| CPC C07D 471/20 (2013.01) [C07D 519/00 (2013.01); C07B 2200/13 (2013.01)] | 20 Claims |
|
1. A method for treating an MC4R-related condition, disease, or disorder in a human, where the method comprises administering to the human in need thereof a compound of Formula I or a pharmaceutically acceptable salt thereof, and wherein the condition, disease, or disorder is selected from cachexia; anorexia or anorexia nervosa; weight loss; failure to thrive; sarcopenia; muscle wasting; muscle weakness; frailty; and malnutrition;
wherein the compound of Formula I is:
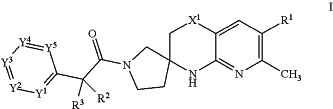 wherein:
R1 is H, halogen, C1-4 alkyl, C1-4 haloalkyl, C3-6 cycloalkyl, 4- to 7-membered heterocycloalkyl, phenyl, or R1a, wherein each of the C3-6 cycloalkyl and 4- to 7-membered heterocycloalkyl optionally substituted with 1, 2, 3, or 4 independently selected C1-4 alkyl, and wherein the phenyl is optionally substituted with 1, 2, 3, or 4 independently selected RB, wherein each RB is halogen, —OH, —CN, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, C1-4 haloalkoxy, C3-4 cycloalkyl, or RB1; or two adjacent RB together with the two ring-forming atoms of the phenyl to which they are attached form a fused 5- or 6-membered heteroaryl, each of which each is optionally substituted with 1, 2, or 3 substituents each independently selected from halogen, —OH, —CN, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, and C1-4 haloalkoxy;
R1a is 5- or 6-membered heteroaryl optionally substituted with 1, 2, 3, or 4 independently selected RA, wherein each RA is halogen, —OH, —CN, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, C1-4 haloalkoxy, C3-4 cycloalkyl, —N(C1-4 alkyl)2, RA1, or (C3-4 cycloalkyl)-C1-4 alkyl-, wherein each of the C1-4 alkyl, C3-4 cycloalkyl, and (C3-4 cycloalkyl)-C1-4 alkyl-is optionally substituted with 1, 2, 3, 4, or 5 substituents each independently selected from halogen, —OH, —CN, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, and C1-4 haloalkoxy; or two adjacent RA together with the two ring-forming atoms of the 5- or 6-membered heteroaryl to which they are attached form a fused benzene ring or a fused 5- or 6-membered heteroaryl or a fused 5- or 6-membered heterocycloalkyl or a fused 5- or 6-membered cycloalkyl, each of which is optionally substituted with 1, 2, or 3 substituents each independently selected from halogen, —OH, —CN, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, and C1-4 haloalkoxy;
RA1 is 5- or 6-membered heteroaryl or 5- or 6-membered heterocycloalkyl, each of which is optionally substituted with 1, 2, or 3 substituents each independently selected from halogen, —OH, —CN, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, and C1-4 haloalkoxy;
RB1 is 5- or 6-membered heteroaryl, each of which is optionally substituted with 1, 2, or 3 substituents each independently selected from halogen, —OH, —CN, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, and C1-4 haloalkoxy;
X1 is C (RX)2, wherein each RX is independently H or C1-4 alkyl;
each of R2 and R3 is independently H, halogen, C1-4 alkyl, C1-4 hydroxyalkyl, C1-4 haloalkyl, (C1-4 alkoxy)-C1-4 alkyl-, C3-4 cycloalkyl, or (C3-4 cycloalkyl)-C1-4 alkyl, wherein each of C3-4 cycloalkyl and (C3-4 cycloalkyl)-C1-4 alkyl-is optionally substituted with 1, 2, 3, 4, or 5 substituents each independently selected from halogen, —OH, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, and C1-4 haloalkoxy;
or R2 and R3 together with the carbon atom to which they are attached form C3-6 cycloalkyl optionally substituted with 1, 2, 3, 4, or 5 substituents each independently selected from halogen, —OH, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, and C1-4 haloalkoxy;
each of Y1, Y2, Y3, Y4, and Y5 is independently CR4 or N, provided that no more than 3 of Y1, Y2, Y3, Y4, and Y5 are N; and
each R4 is independently H, halogen, —OH, —CN, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, C1-4 haloalkoxy, —N(C1-2 alkyl)2, C3-4 cycloalkyl, or (C3-4 cycloalkyl)-C1-4 alkyl-, wherein each of the C1-4 alkyl, C3-4 cycloalkyl, and (C3-4 cycloalkyl)-C1-4 alkyl-is optionally substituted with 1, 2, 3, 4, or 5 substituents each independently selected from halogen, —OH, —CN, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, and C1-4 haloalkoxy.
|