| CPC A61M 39/0606 (2013.01) [A61M 25/09 (2013.01); A61M 29/00 (2013.01); A61M 2039/062 (2013.01); A61M 60/135 (2021.01); A61M 2205/0216 (2013.01); A61M 2207/00 (2013.01)] | 18 Claims |
|
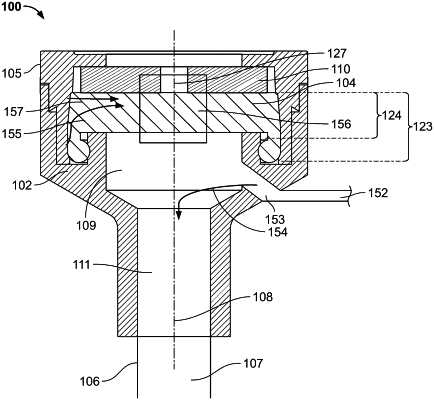
1. An introducer sheath assembly for percutaneously delivering a medical device that maintains hemostasis in a patient, the introducer sheath assembly comprising:
a sheath body; and
a sheath hub assembly coupled to the sheath body, the sheath hub assembly comprising a hub, a hub cap, a hemostasis valve and, optionally, a foam,
wherein the hemostasis valve comprises a single component comprising a valve portion and a frame portion, the frame portion defining a perimeter of the hemostasis valve and having a thickness that is greater than a thickness of the valve portion, the valve portion having a plurality of offset slits formed through the thickness of the valve portion,
wherein the hub has a valve seating feature formed therein, the valve seating feature being a channel having an inner wall and an outer wall into which is received a portion of the hemostasis valve and a portion of the hub cap.
|