| CPC A61M 1/3687 (2013.01) [A61M 1/16 (2013.01); A61M 1/3486 (2014.02); A61M 1/3679 (2013.01); B01D 63/031 (2022.08); B01D 63/034 (2022.08); B01D 69/148 (2013.01); B01D 71/383 (2022.08); B01D 71/421 (2022.08); B01D 71/68 (2013.01); B01D 71/76 (2013.01); B01J 31/003 (2013.01); B01D 2323/21825 (2022.08); B01D 2323/2187 (2022.08)] | 20 Claims |
|
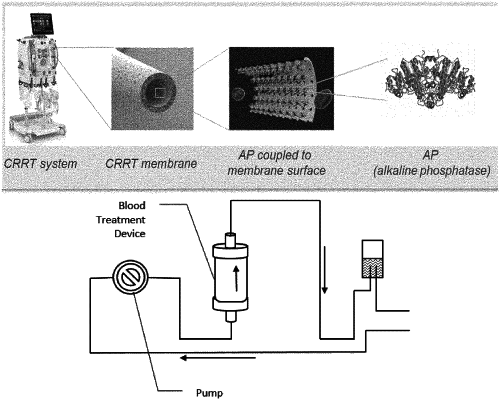
1. A blood treatment device configured to dephosphorylate one or more substances selected from the group consisting of extracellular adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), lipopolysaccharide (LPS), and combinations thereof, in the blood of a patient in need thereof in an extracorporeal blood circuit, wherein the device comprises a matrix having alkaline phosphatase (AP) immobilized thereon.
|
|
14. A method for treating an infection in a patient, said method comprising the step of administering the blood treatment device according to claim 1 to the patient, wherein the blood treatment device transports blood from the vascular system of the patient to the blood treatment device and returns treated blood from the blood treatment device to the patient.
|