| CPC A61K 35/747 (2013.01) [A61K 31/702 (2013.01); A61K 35/745 (2013.01); A61P 35/00 (2018.01); C07K 16/2818 (2013.01); C07K 16/2827 (2013.01); A61K 2035/115 (2013.01)] | 2 Claims |
|
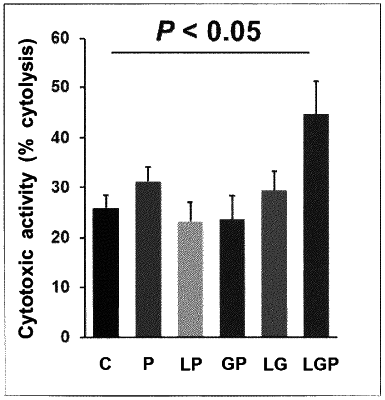
1. A method for potentiating an antitumor effect of an immune checkpoint inhibitor, the method comprising:
administering an effective amount of a probiotic, a prebiotic, and an immune checkpoint inhibitor to a subject in need thereof,
wherein the probiotic comprises Lactobacillus casei YIT9029 (FERM BP-1366) and Bifidobacterium breve YIT12272 (FERM BP-11320),
the prebiotic is a galactooligosaccharide, and
the immune checkpoint inhibitor is an anti-PD-1 antibody.
|