| CPC A61K 31/522 (2013.01) [A61K 9/0019 (2013.01); A61K 39/395 (2013.01); A61P 43/00 (2018.01)] | 9 Claims |
|
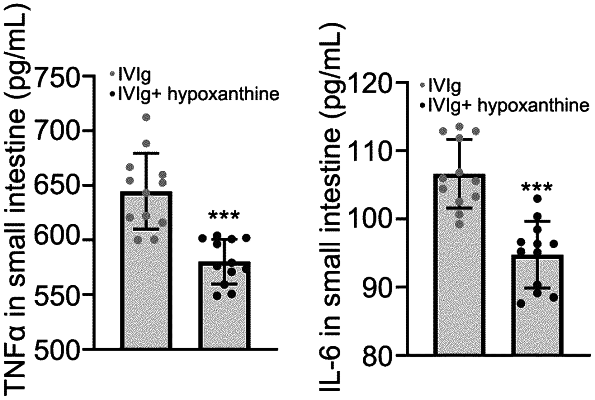
1. A combination drug for the treatment of radiation injuries for a subject in need thereof, comprising hypoxanthine and human immunoglobulin (HIg), wherein:
said HIg is selected from intravenous immunoglobulin (IVIg), intramuscular immunoglobulin (IMIg), subcutaneous immunoglobulin (SCIg), and mixtures thereof; and
the mass of hypoxanthine is 50 mg/kg calculated based on the subject's weight; and
the mass ratio of hypoxanthine to HIg is from 1:1 to 1:11.
|