| CPC A61K 31/4375 (2013.01) [A61K 9/2054 (2013.01); A61K 31/4045 (2013.01); A61K 31/47 (2013.01)] | 22 Claims |
|
1. A method of treating cystic fibrosis comprising daily administration of:
(a) 250 mg of Compound I:
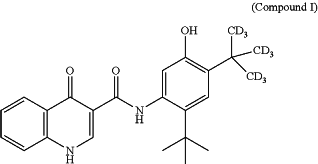 or an equivalent amount of a pharmaceutically acceptable salt thereof; and
(b) 21.24 mg Compound II calcium salt hydrate Form D:
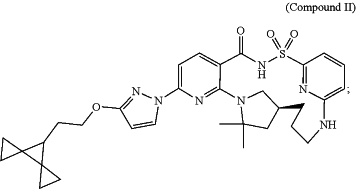 and
(c) 100 mg of Compound III:
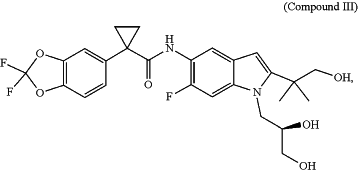 or an equivalent amount of a pharmaceutically acceptable salt thereof.
|