| CPC C07D 403/04 (2013.01) [A01N 43/54 (2013.01); A01N 43/56 (2013.01); A01N 43/58 (2013.01); A01N 43/80 (2013.01); A01N 43/90 (2013.01); C07D 207/325 (2013.01); C07D 207/337 (2013.01); C07D 207/34 (2013.01); C07D 401/04 (2013.01); C07D 401/14 (2013.01); C07D 409/14 (2013.01); C07D 413/04 (2013.01); C07D 471/04 (2013.01); C07D 487/04 (2013.01)] | 21 Claims |
|
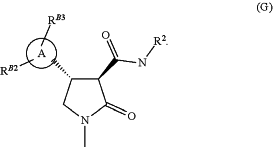
1. A process for the manufacture of a compound of formula (G)
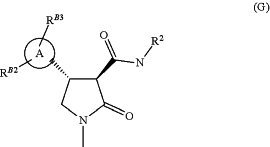 wherein ring A is a di-substituted pyrazole, substituted on a ring nitrogen by RB2 and substituted on a ring carbon by RB3;
RB2 is C1-C3 alkyl or C1-C3fluoroalkyl,
RB3 is halogen, C1-C3fluoroalkyl, C1-C3haloalkoxy, C1-C3alkoxy, C1-C3haloalkyl, or C1-C3alkyl;
R2 is an aryl or heteroaryl ring optionally substituted by 1 to 3 R25; and
each R25 is independently halogen, C1-C6alkyl, C1-C6haloalkyl, C1-C6alkoxy, C1-C6haloalkoxy, cyano, nitro, C1-C6alkylthio, C1-C6alkylsulphinyl, or C1-C6alkylsulphonyl; said process comprising:
(i) reacting a compound of formula (A)
 wherein ring A, RB2 and RB3 are as defined previously, and Hal is iodo, bronco or chloro, with 1-dimethylamino-2-nitroethylene to form a compound of formula (B)
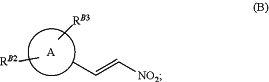 (ii) reacting the compound of formula (B) from step (i) with a malonate in solvent, under enantioselective nickel catalysis to give a compound of formula (C)
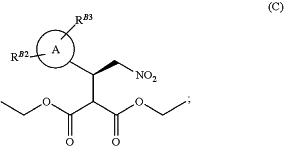 (iii) reacting the compound of formula (C) from step (ii) with a reducing agent in the presence of a catalyst to give a compound of formula (D)
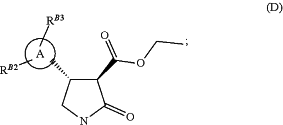 (iv) hydrolysing the compound of formula (D) from step (iii) in an aqueous hydroxide/ethanol mixture to give the compound of formula (E)
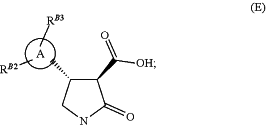 (v) methylating the lactam nitrogen of the compound of formula (E) formed in step (iv) using excess base in solvent, to form a compound of formula (1′)
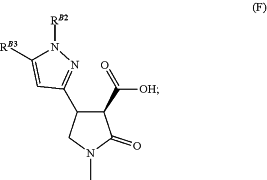 (vi) coupling the compound of formula (F) from step (v) with an aniline of formula R2—NH2 wherein R2 is as defined above under standard amide coupling conditions, to yield a compound of formula (G)
 |