| CPC C07D 213/42 (2013.01) [A61K 51/0455 (2013.01); A61K 51/0497 (2013.01); A61P 35/00 (2018.01); C07B 59/002 (2013.01); C07D 213/82 (2013.01)] | 34 Claims |
|
1. A method of synthesizing 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid [18F]DCFPyL),
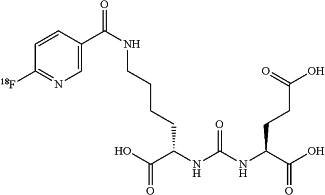 the method comprising:
(i) radiofluorinating a DCFPyL precursor comprising ester moiety protecting groups to form a radiofluorinated DCPFPyL precursor;
(ii) deprotecting the ester moiety protecting groups of the radiofluorinated DCPFPyL precursor of step (i) with phosphoric acid to form [18F]DCFPyL in a reaction mixture; and
(iii) purifying the [18F]DCFPyL from the reaction mixture of step (ii) to provide [18F]DCFPyL.
|