| CPC C07C 211/22 (2013.01) [A61K 47/18 (2013.01); A61K 48/00 (2013.01); A61P 29/00 (2018.01); A61P 35/00 (2018.01); C07C 59/01 (2013.01); C07C 215/08 (2013.01); C07C 219/04 (2013.01); C07C 229/02 (2013.01); C07C 229/10 (2013.01); C07C 229/12 (2013.01); C07C 233/00 (2013.01); C07C 243/00 (2013.01); C07C 243/10 (2013.01); C07C 243/14 (2013.01); C07C 243/16 (2013.01); C07C 243/26 (2013.01); C07C 243/28 (2013.01); C07C 279/04 (2013.01); C07C 333/10 (2013.01); C07D 233/64 (2013.01); C07D 295/13 (2013.01); C07D 295/15 (2013.01); C12N 15/113 (2013.01); C12N 2310/14 (2013.01)] | 24 Claims |
|
1. A cationic lipid which is represented by the structure of formula (I), formula (II) or formula (IIIA), or salts, hydrates, solvates, polymorphs, optical isomers, geometrical isomers, enantiomers, diastereomers, and mixtures thereof, wherein the structure of formula (I), formula (II) and formula (IIIA) is represented below:
Formula (I):
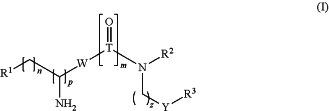 wherein
Y is O or NH;
T is C or S;
W is a bond or NH;
R1 is selected from the group consisting of:
(a) NR4R5 wherein R4 and R5 are each independently a C1-C4 alkyl; or R4 and R5 together with the nitrogen to which they are attached form a 5 or 6 membered heterocyclic or heteroaromatic ring, optionally containing one or more additional heteroatoms selected from the group consisting of O, N and S; or NR4R5 represent a guanidine group (—NHC(═NH)NH2);
(b) the side chain of histidine or arginine; and
(c) a 5 or 6 membered heterocyclic or heteroaromatic ring containing one or more heteroatoms selected from the group consisting of O, N and S;
R2 and R3 are selected from the group consisting of:
(a) C10-C22 alkyl;
(b) C10-C22 alkenyl;
(c) C10-C22 alkynyl;
(d) C4-C10 alkylene-Z— C4-C22 alkyl; and
(e) C4-C10 alkylene-Z— C4-C22 alkenyl;
Z is —O—C(═O)—, —C(═O)—O— or —O—;
n is 0, 1, 2, 3, 4, 5 or 6;
m is 0 or 1;
p is 0 or 1; and
z is 0 or 2;
Formula (II):
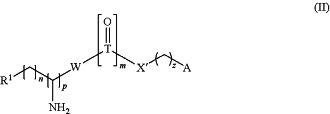 wherein
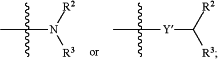
A is
 X′ is O or NH;
Y′ is O or NH;
provided that when A is
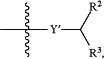 X′ and Y′ cannot both be O;
T is C or S;
W is a bond, O, NH or S;
R1 is selected from the group consisting of:
(a) NR4R5 wherein R4 and R5 are each independently a C1-C4 alkyl; or R4 and R5 together with the nitrogen to which they are attached form a 5 or 6 membered heterocyclic or heteroaromatic ring, optionally containing one or more additional heteroatoms selected from the group consisting of O, N and S; or NR4R5 represent a guanidine group (—NHC(═NH)NH2);
(b) the side chain of histidine or arginine; and
(c) a 5 or 6 membered heterocyclic or heteroaromatic ring containing one or more heteroatoms selected from the group consisting of O, N and S;
R2 and R3 are selected from the group consisting of:
(a) C10-C22 alkyl;
(b) C12-C22 alkenyl;
(c) C10-C22 alkynyl;
(d) C4-C10 alkylene-Z—C4-C22 alkyl; and
(e) C4-C10 alkylene-Z—C4-C22 alkenyl;
Z is —O—C(═O)—, —C(═O)—O— or —O—;
n is 0, 1, 2, 3, 4, 5 or 6;
m is 0 or 1;
p is 0 or 1; and
z is 0 or 2;
Formula (IIIA):
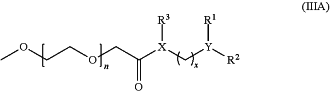 wherein
X and Y are each independently O, N or NH, wherein X and Y cannot both be O;
each of R1, R2 and R3 is independently absent or a C10-C22 alkyl, a C10-C22 alkenyl or a C10-C22 alkynyl, wherein the alkyl group is unsubstituted or substituted by one or more groups selected from halogen, hydroxy, alkoxy carbonyl, amido, alkylamido, dialkylamido, nitro, amino, alkylamino, dialkylamino, carboxyl, thio and thioalkyl;
n is an integer between 1 and 30; and
x is 0 or 2.
|