| CPC A61L 33/0023 (2013.01) [A61L 33/062 (2013.01); C08L 5/10 (2013.01); A61L 2300/236 (2013.01); A61L 2300/42 (2013.01)] | 32 Claims |
|
1. An anticoagulant surface which surface has covalently bound thereto a plurality of fragments of heparin, wherein said fragments consist of 5-18 saccharide units and at least some of said plurality of fragments comprise polysaccharide sequence A:
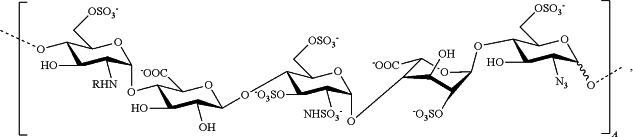 wherein R=Ac or SO3−
which surf ace catalyses the inhibition of FIIa and FXa by AT, and wherein the surface has a heparin fragment concentration of at least 1 μg/cm2.
|