| CPC A61K 9/06 (2013.01) [A61K 9/02 (2013.01); A61K 31/191 (2013.01); A61K 31/702 (2013.01); A61K 31/7016 (2013.01); A61K 33/40 (2013.01); A61K 35/747 (2013.01)] | 20 Claims |
|
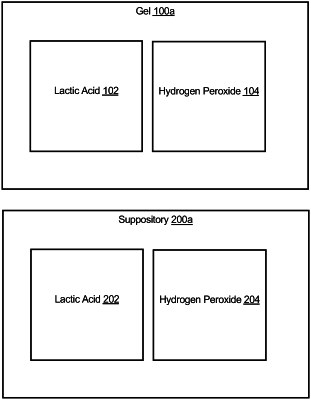
1. A gel, the gel consisting of:
between 50 and 150 millimolar of lactic acid concentrate;
between 0.5 and 10 millimolar of hydrogen peroxide concentrate, wherein the between 50 and 150 millimolar of lactic acid concentrate is combined with the between 0.5 and 10 millimolar of hydrogen peroxide concentrates; and
optionally, at least one of a probiotic, a prebiotic, a vitamin and a moisturizer.
|