| CPC C08F 2/50 (2013.01) [C08F 2/44 (2013.01); C09D 11/101 (2013.01)] | 16 Claims |
|
1. A photocurable composition comprising:
a) from 50 to 99.9% by weight of at least one ethylenically unsaturated compound;
b) from 0.1 to 35% by weight, of the solids content of at least one compound of formula (I) and/or (II)
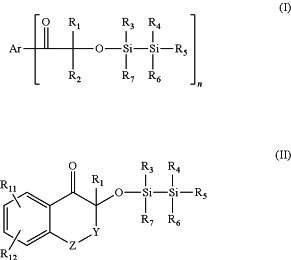 wherein:
n is 1 or 2;
when n is 1, Ar is selected form:
a C6-C12 aryl which is unsubstituted or substituted by one or more substituents selected from the group consisting of halogens, —CN, —COOH, —OH, C1-C18 alkyl, —Oalkyl, —Ophenyl, —SH, —Salkyl, —Salkoxy, —Sphenyl, —SOalkyl, —SO2alkyl, —SO2alkoxy, —SO2phenyl, —COOalkyl, SO2NH2, —SO2NHalkyl, —SO2N(alkyl)2, —NHalkyl, —NHalkoxy, —N(alkyl)2, morfolino, piperidino, piperazino, —N(alkoxy)2, —NHCOalkyl, —NHCOphenyl; and
one of the following groups pyridyl, thienyl, 2-methylthienyl, pyrryl, furyl, indanyl, imidazolyl, thiazolyl, oxazolyl, tetrahydronaphthyl, naphthyl, benzothienyl, benzopyrryl, benzofuryl, benzoimidazolyl, benzothiazolyl, benzoxazolyl, 3,4-ethylenedioxythiophene, carbazolyl, N-alkylcarbazolyl, thianthrenyl, phenoxathiinyl, phenothiazinyl, phenoxazinyl, 5,10-dihydrophenazinyl; all said groups being optionally substituted by one or more electrodonating groups;
when n is 2, Ar is selected from a C6-C12 arylene group, a C6-C12 heteroarylene group, and a -arylene-T-arylene- group selected from a -phenylene-T-phenylene- group and a trimethyl-phenyl-indane group selected from the one of formula
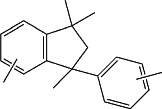 all said groups being optionally substituted by one or more electrodonating groups;
Y is selected from a direct bond, —CH2—, —CH2—CH2—, —O—, —S— and -Nalkyl;
Z is selected from a direct bond, —O—, —S—, —SO2—, -Nalk, —CH2— and —C(CH3)2;
T is selected from a direct bond, —O—, —S—, —SO2—, —CH2—, —CH2CH2—, —CH2OCH2— and —CH═CH—;
R1 is selected from, C1-C8 alkyl which is unsubstituted or substituted by C2-C8acyloxy, —NR8R9, —COOalkyl, or —CN, or represents C3-C5alkenyl, C5-C12cycloalkyl, phenyl and benzyl;
R2 is selected from R1 or R2 represents a —CH2CH2R10 group; or R1 and R2, together with the carbon atom to which they are bound, represent C5-C12cycloalkyl, C2-C8-alkylene or C3-C9oxa- or aza-alkylene;
R3, R4, R5, R6 and R7, each independently, are selected from
phenyl, C1-C8alkyl, both being unsubstituted or substituted by —OH, —Oalkyl, C2-C8acyloxy, —NR3R9, —COOalkyl or CN; and
Oalkyl;
R8 and R9, each independently, are selected from C1-C18alkyl which is unsubstituted or substituted by —OH, —Oalkyl, or —CN;
R10 is selected from —CONH2, —CONHalkyl, —CON(alkyl)2 and —P(O)(Oalkyl)2
R11 is selected from hydrogen, halogen, —CN, —COOH, —OH, C1-C18 alkyl, —Oalkyl, —Oalkoxy, —Ophenyl, —SH, —Salkyl, —Salkoxy, —Sphenyl, —SOalk, —SO2alkyl, —SO2alkoxy, —SO2phenyl, —COOalkyl, SO2NH2, —SO2NHalkyl, —SO2N(alkyl)2, —NHalkyl, —NHalkoxy, —N(alkyl)2, morfolino, piperidino, piperazino, —N(alkoxy)2, —NHCOalkyl and —NHCOphenyl;
R12 has one of the meanings assigned to R11;
or R11 and R12, together with the carbon atoms to which they are bound, represent C5-C12cycloalkyl or C6-C12 aryl;
c) from 0.01 to 15% by weight of the solids content of at least one photosensitizer from the group of aromatic carbonyl compounds having triplet energy of 225-310 kJ/mol.
|