| CPC C07K 5/0215 (2013.01) [A61P 37/06 (2018.01); C07K 11/02 (2013.01); A61K 38/00 (2013.01)] | 12 Claims |
|
1. A compound of Formula (I):
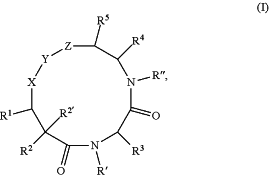 wherein
X is
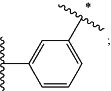 Y is O;
Z is
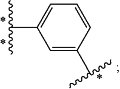  is the point of attachment to —C(R1)— moiety;
 is the point of attachment to Y;
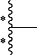 is the point of attachment to —C(R5)— moiety;
R is H;
R2 is independently selected at each occurrence thereof from the group consisting of H, C1-6 alkyl, arylalkyl, —NR6R7, —NHC(O)R8, —NHS(O)2R8, and —NHC(O)(CH2)nNR6R7;
R2′ is H or C1-6 alkyl;
R3 is independently selected at each occurrence thereof from the group consisting of H, C1-6 alkyl, —(CH2)nNR6R7, —CH2C(O)NR6R7, —CH2C(O)OH, and arylalkyl, wherein C1-6 alkyl or arylalkyl can be optionally substituted from 1 to 3 times with halogen, C1-6 alkoxy, —O-aryl, and CF3;
R4 is selected from the group consisting of R9, —C(O)R9, —C(O)NH(CRaRb)nR8, —C(O)N(Me)(CRaRb)nR8, —C(O)CH2Ph, —C(O)OR9, —CH2NHR8, and —C(O)NR6R7;
R5 is H;
R6 and R7 are each independently selected from the group consisting of H, C1-6 alkyl, C3-8 cycloalkyl, and C3-12 cycloalkylalkyl, or, wherein C3-8 cycloalkyl and C3-12 cycloalkylalkyl can be optionally substituted from 1 to 3 times with CF3;
or R6 and R7 are taken together with the nitrogen to which they are attached to form a piperidine, pyrrolidine, morpholine ring, piperazine, oxazolidine, or isothiazolidine, wherein piperidine, pyrrolidine, morpholine, piperazine, oxazolidine, or isothiazolidine ring can be optionally substituted 1 to 3 times with halogen, C1-6 alkyl, aryl, ═O, C3-8 cycloalkyl, or non-aromatic heterocycle;
R8 is selected from the group consisting of H, OH, CF3, CHF2, C1-12 alkyl, C3-8 cycloalkyl, C3-12 cycloalkylalkyl, monocyclic or bicyclic aryl, arylalkyl, heteroaryl, heterocyclyl, and non-aromatic heterocycle, wherein C1-12 alkyl, C3-8 cycloalkyl, C3-12 cycloalkylalkyl, monocyclic or bicyclic aryl, arylalkyl, heteroaryl, heterocyclyl, and non-aromatic heterocycle can be optionally substituted from 1 to 3 times with OH, halogen, C1-6 alkyl, C1-6 alkoxy, CHF2, CF3, —S(O)2Me;
R9 is selected from the group consisting of CF3, CHF2, C2-12 alkyl, C3-8 cycloalkyl, C3-12 cycloalkylalkyl, C2-12 alkoxy, monocyclic or bicyclic aryl, arylalkyl, and heteroaryl, wherein C1-12 alkyl, C3-8 cycloalkyl, C3-12 cycloalkylalkyl, monocyclic or bicyclic aryl, arylalkyl, and heteroaryl, can be optionally substituted from 1 to 3 times with OH, halogen, C1-6 alkyl, C1-6 alkoxy, CHF2, CF3, —S(O)2Me;
Ra and Rb are each independently selected from the group consisting of H and C1-6 alkyl;
R′ and R″ are each independently selected from the group consisting of H and C1-6 alkyl;
n is 0, 1, 2, 3, or 4; and
m is 3,
with the proviso that i) R2 is not NH2 and ii) R4 is not
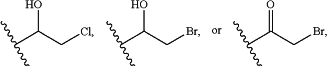 or an oxide thereof, a pharmaceutically acceptable salt thereof, or a solvate thereof.
|