| CPC C07D 491/22 (2013.01) [C07D 471/14 (2013.01)] | 34 Claims |
|
1. A compound of Formula III, or a stereoisomer, mixture of stereoisomers, or pharmaceutically acceptable salt thereof:
A1-L1-B1 III
wherein:
L1 is a covalent bond or a linking moiety;
B1 is a nuclear receptor-targeting epitope selected from the group consisting of;
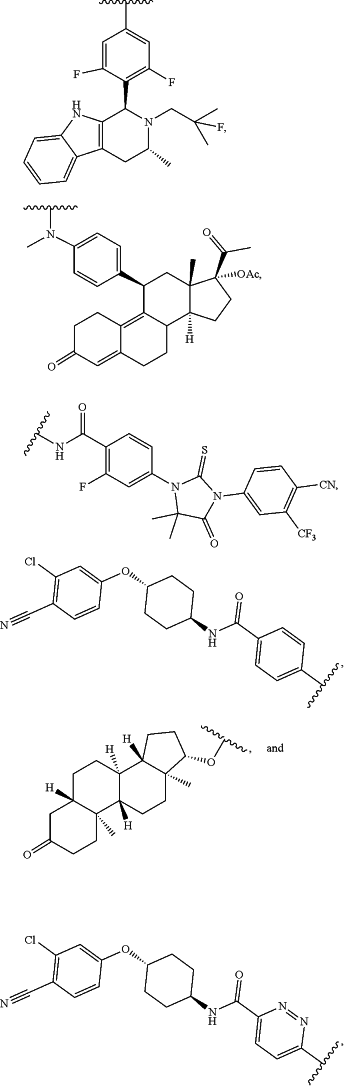 wherein the wavy bond refers to the point of connection to L1; and
A1 is of Formula IA:
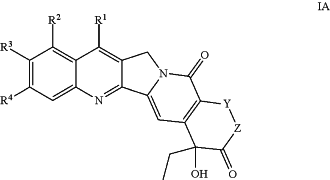 wherein:
Y is a bond, —CH2—, or —CH2—CH2—;
Z is a bond or O;
R1, R2, R3, and R4, are each independently hydrogen, halo, cyano, nitro, —OR15, —SR15, —NR15R16, C1-12 alkyl, C2-12 alkenyl, C2-12 alkynyl, C3-10 cycloalkyl, heterocyclyl, aryl, heteroaryl, —C(═O)R15, —C(═O)OR15, —OC(═O)R15, —C(═O)NR15R16, —NR15C(═O)R16, —NR15C(═O)OR16, —S(═O)1-2R15, —S(═O)1-2NR15R16, —NR15S(═O)1-2R16, —Si(R15)3, or —C═NOR15, wherein each alkyl, alkenyl, alkynyl, cycloalkyl, heterocyclyl, aryl and heteroaryl of R1, R2, R3, and R4, are independently optionally substituted with one or more R10 as valency permits;
or R1 and R2 are taken together with the atoms to which they are attached to form a C3-10 cycloalkyl, heterocyclyl, aryl, or heteroaryl, optionally substituted with one or more R10 as valency permits;
or R2 and R3 are taken together with the atoms to which they are attached to form a C3-10 cycloalkyl, heterocyclyl, aryl, or heteroaryl, optionally substituted with one or more R10 as valency permits;
or R3 and R4 are taken together with the atoms to which they are attached to form a C3-10 cycloalkyl, heterocyclyl, aryl, or heteroaryl, optionally substituted with one or more R10 as valency permits;
each R10 is independently halo, cyano, nitro, —OR17, —SR17, —SF5, —NR17R18, C1-12 alkyl, C2-12 alkenyl, C2-12 alkynyl, C3-10 cycloalkyl, heterocyclyl, aryl, heteroaryl, —C(═O)R17, —C(═O)OR17, —OC(═O)OR17, —OC(═O)R17, —C(═O)NR17R18, —OC(═O)NR17R18, —NR17C(═O)NR17R18, —S(═O)1-2R17, —S(═O)1-2NR17R18, —NR17S(═O)1-2R18, —NR17S(═O)1-2NR17R18, —NR17C(═O)R18, —NR17C(═O)OR18, —Si(R17)3, or —C═NOR17, wherein each alkyl, alkenyl, alkynyl, cycloalkyl, heterocyclyl, aryl and heteroaryl of R10 are independently optionally substituted with one or more halo or C1-12 alkyl optionally substituted by oxo, halo, hydroxyl, or amino as valency permits; and
each R15 and R16 is independently hydrogen, C1-12 alkyl, C2-12 alkenyl, C2-12 alkynyl, or C3-12 cycloalkyl, wherein each alkyl, alkenyl, alkynyl, or cycloalkyl is optionally independently substituted with oxo, halo, hydroxyl, or amino as valency permits; or R15 and R16 are taken together with the atoms to which they are attached to form heterocyclyl optionally substituted by halo or C1-12 alkyl optionally substituted by oxo, halo, hydroxyl, or amino; and
each R17 and R18 is independently hydrogen, C1-12 alkyl, C2-12 alkenyl, C2-12 alkynyl, or C3-12 cycloalkyl, wherein each C1-12 alkyl, C2-12 alkenyl, C2-12 alkynyl, or C3-12 cycloalkyl is optionally substituted with oxo, halo, hydroxyl, or amino as valency permits; or R17 and R18 are taken together with the atoms to which they are attached to form heterocyclyl optionally substituted by halo or C1-12 alkyl optionally substituted by oxo, halo, hydroxyl, or amino;
wherein one or more atoms in one of R1, R2, and R3 of Formula IA is replaced by a direct covalent bond to L1.
|