| CPC C07D 487/10 (2013.01) [C07D 239/42 (2013.01); C07D 401/04 (2013.01); C07D 403/04 (2013.01); C07D 403/12 (2013.01); C07D 405/04 (2013.01); C07D 405/12 (2013.01); C07D 405/14 (2013.01); C07D 409/14 (2013.01); C07D 413/04 (2013.01); C07D 413/12 (2013.01); C07D 413/14 (2013.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07D 471/10 (2013.01); C07D 487/04 (2013.01); C07D 487/08 (2013.01); C07D 493/04 (2013.01); C07D 493/10 (2013.01); C07D 495/04 (2013.01); C07D 498/04 (2013.01); C07D 498/10 (2013.01)] | 20 Claims |
|
1. A compound of Formula (I-h):
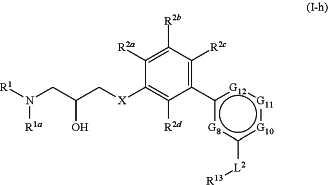 or a pharmaceutically acceptable salt thereof, wherein:
X is —O—, —S—, or —CH2—;
R1 and R1a are each independently hydrogen or optionally substituted C1-4 aliphatic;
each of R2a, R2b, R2c, and R2d is independently hydrogen, halogen, —CN, —NO2, —C(═O)RA2, —C(═O)ORA2, —C(═O)N(RA2)2, —ORA2, —SRA2, —N(RA2)2, —S(═O)RA2, —S(═O)2RA2, optionally substituted alkyl, optionally substituted alkenyl, optionally substituted alkynyl, optionally substituted carbocyclyl, or optionally substituted heterocyclyl, wherein each instance of RA2 is independently hydrogen, optionally substituted alkyl, optionally substituted alkenyl, optionally substituted alkynyl, optionally substituted carbocyclyl, optionally substituted heterocyclyl, optionally substituted aryl, or optionally substituted heteroaryl, or two RA2 groups attached to the same nitrogen atom are joined to form an optionally substituted heterocyclyl or optionally substituted heteroaryl ring;
G8 is C—R8 or N;
G10 is C—R10 or N;
G11 is C—R11 or N;
G12 is C—R12 or N;
provided at least two instances of G8, G10, G11, or G12 are N;
each instance of R8, R10, R11, and R12 is independently selected from the group consisting of hydrogen, halo, —CN, —NO2, —C(═O)R′, —C(═O)OR′, —C(═O)N(R′)2, optionally substituted alkyl, and -L1-R3;
each instance of R′ is independently hydrogen, optionally substituted alkyl, optionally substituted alkenyl, optionally substituted alkynyl, optionally substituted carbocyclyl, optionally substituted heterocyclyl, optionally substituted aryl, or optionally substituted heteroaryl, or two R′ groups attached to the same nitrogen atom are joined to form an optionally substituted heterocyclyl ring or optionally substituted heteroaryl ring;
each instance of L1 and L2 is independently a bond, —O—, —N(RL)—, —S—, —C(O)—, —C(O)O—, —C(O)S—, —C(O)N(RL)—, —C(O)N(RL)N(RL)—, —OC(O)—, —OC(O)N(RL)—, —NRLC(O)—, —NRLC(O)N(RL)—, —NRLC(O)N(RL)N(RL)—, —NRLC(O)O—, —SC(O)—, —C(═NRL)—, —C(═NNRL)—, —C(═NORL)—, —C(═N RL)N(RL)—, —NRLC(═NRL)—, —C(S)—, —C(S)N(RL)—, —NRLC(S)—, —S(O)—, —OS(O)2—, —S(O)2O—, —SO2—, —N(RL)SO2—, —SO2N(RL)—, —N(RL)SO2N(RL)—, or an optionally substituted C1-10 saturated or unsaturated hydrocarbon chain, wherein one or more moieties selected from the group consisting of —O—, —N(RL)—, —S—, —C(O)—, —C(O)O—, —C(O)S—, —C(O)N(RL)—, —C(O)N(RL)N(RL)—, —OC(O)—, —OC(O)N(RL)—, —NRLC(O)—, —NRLC(O)N(RL)—, —NRLC(O)N(RL)N(RL)—, —NRLC(O)O—, —SC(O)—, —C(═NRL)—, —C(═NNRL)—, —C(═NORL)—, —C(═N RL)N(RL)—, —NRLC(═NRL)—, —C(S)—, —C(S)N(RL)—, —NRLC(S)—, —S(O)—, —OS(O)2—, —S(O)2O—, —SO2—, —N(RL)SO2—, —SO2N(RL)—, and —N(RL)SO2N(RL)— is optionally and independently present between two carbon atoms of the hydrocarbon chain, and optionally and independently present at one or both ends of the hydrocarbon chain;
each RL is independently hydrogen, optionally substituted alkyl, or a nitrogen protecting group, or RL and R3 taken together form an optionally substituted heterocyclyl or optionally substituted heteroaryl ring, or RL and R13 taken together form an optionally substituted heterocyclyl or optionally substituted heteroaryl ring;
R3 is hydrogen, optionally substituted alkyl, optionally substituted alkenyl, optionally substituted alkynyl, optionally substituted carbocyclyl, optionally substituted heterocyclyl, optionally substituted aryl, or optionally substituted heteroaryl, provided when R3 is hydrogen, then L1 is not a bond; and
R13 is optionally substituted carbocyclyl, optionally substituted heterocyclyl, optionally substituted heteroaryl, or aryl unsubstituted or substituted with one or more substituents independently selected from the group consisting of halogen, —CN, —NO2, —N3, —SO2H, SO3H, —OH, —ORaa, —N(Rbb)2, —SH, —SRaa, —C(═O)Raa, —CO2H, —CHO, —CO2Raa, —OC(═O)Raa, —OCO2Raa, —C(═O)N(Rbb)2, —OC(═O)N(Rbb)2, C1-4 alkyl, C1-4 perhaloalkyl, C2-4 alkenyl, and C2-4 alkynyl;
wherein:
each instance of Raa is, independently, C1-4 alkyl; and
each instance of Rbb is, independently, hydrogen or C1-4 alkyl, or two Rbb groups are joined to form a 3-6 membered heterocyclyl or 5-6 membered heteroaryl ring.
|