| CPC C07D 487/04 (2013.01) [A61K 51/0459 (2013.01); G01N 33/57492 (2013.01); G01N 2333/7158 (2013.01)] | 14 Claims |
|
1. A compound having formula I
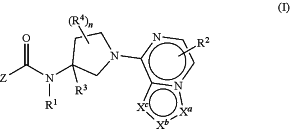 or a pharmaceutically acceptable salt, hydrate, N-oxide, isotopically enriched or enantiomerically enriched version or a rotamer thereof, wherein
each of ring vertices Xa, Xb and Xc is independently selected from the group consisting of N, NH, N(R2), O, CH and C(R2);
the subscript n is 0, 1 or 2;
Z is selected from the group consisting of
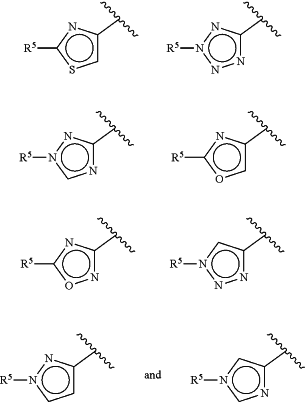 R1 is a member selected from the group consisting of H and C1-8 alkyl, wherein the alkyl portion is optionally substituted with halogen, —NRaRb, —ORa, —CO2Ra and —CONRaRb;
each R2 is independently selected from the group consisting of H and C1-4 alkyl;
R3 is a member selected from the group consisting of H, C1-8 alkyl, C1-8 haloalkyl, C1-8 hydroxyalkyl, —CO2Ra, —X—CO2Ra, —CONRaRb and —X—CONRaRb;
each R4, when present, is a member independently selected from the group consisting of C1-8 alkyl, C1-8 haloalkyl, C1-8 hydroxyalkyl, —ORa, —CO2Ra, —X—CO2Ra, —NRaRb, —CONRaRb and —X—CONRaRb;
R5 is selected from the group consisting of substituted aryl, heteroaryl, cycloalkyl, and heterocycloalkyl optionally further substituted with 1-3 Ra;
each Ra and Rb is independently selected from the group consisting of hydrogen, hydroxyl, halogen, cyano, C1-8 alkyl, C1-8 alkoxy, C1-8 haloalkyl, C3-6 cycloalkyl, C3-6 cycloalkylalkyl, amino, C1-8 alkylamino, di C1-8 alkylamino, carboxamide, carboxy C1-4 alkyl ester, carboxylic acid, and —SO2— C1-8 alkyl;
each X is a C1-4 alkylene linking group or a linking group having the formula —(CH2)mO(CH2)p—, wherein the subscripts m and p are integer of from 0 to 5, and m+p is from 0 to 6, wherein any of the methylene portions of X are optionally substituted with one or two methyl groups.
|