| CPC C07C 227/18 (2013.01) [C07C 227/40 (2013.01)] | 10 Claims |
|
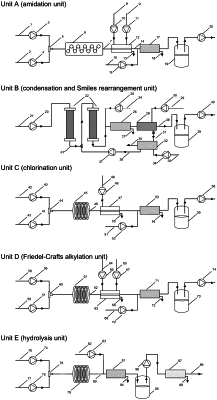
1. A method for preparing diclofenac sodium using a continuous-flow reaction system, the continuous-flow reaction system comprising a first unit, a second unit, a third unit, a fourth unit and a fifth unit successively connected in series; the first unit comprising a first mixer, a first microchannel reactor and a first storage tank connected in sequence; the second unit comprising a second mixer, a second microchannel reactor, a third microchannel reactor, a filter and a second storage tank; the third unit comprising a third mixer, a fourth microchannel reactor and a third storage tank connected in sequence; the fourth unit comprising a fourth mixer, a fifth microchannel reactor, and a fourth storage tank connected in sequence; the fifth unit comprising a fifth mixer and a sixth microchannel reactor; and the method comprising:
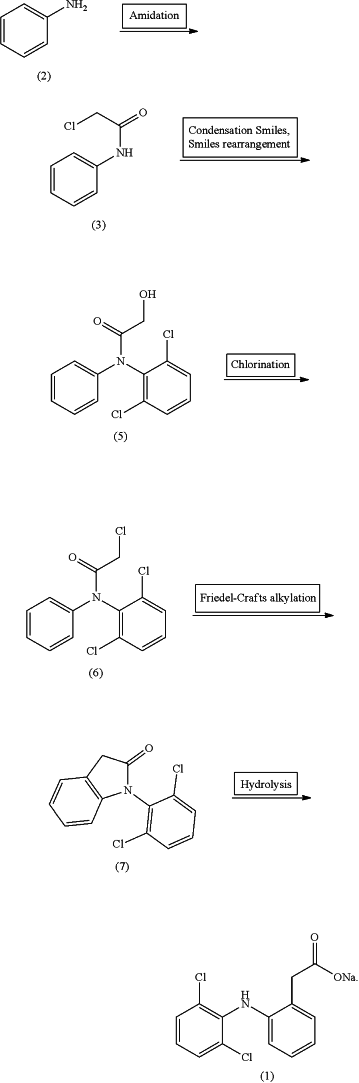
(S1) respectively feeding a mixed solution of chloroacetic acid and a first catalyst, and an organic solution of aniline to the first mixer for mixing to obtain a first mixture; and allowing the first mixture to flow out of the first mixer and enter the first microchannel reactor for amidation reaction;
(S2) subjecting a reaction mixture flowing out of the first microchannel reactor to continuous quenching, liquid-liquid extraction and separation to collect an organic phase;
(S3) concentrating the organic phase obtained in step (S2) to obtain 2-chloro-N-phenylacetamide (3) followed by dissolving with a first organic solvent to obtain a 2-chloro-N-phenylacetamide (3) solution; and transporting the 2-chloro-N-phenylacetamide (3) solution to the first storage tank;
(S4) respectively transporting the 2-chloro-N-phenylacetamide (3) solution in the first storage tank, and a mixed solution of 2,6-dichlorophenol and a phase transfer catalyst to the second mixer to for mixing to obtain a second mixture; and allowing the second mixture to flow out of the second mixer and enter the second microchannel reactor and the third microchannel reactor in sequence for continuous condensation and Smiles rearrangement; wherein the second microchannel reactor and the third microchannel reactor are both filled with a basic catalyst;
(S5) concentrating a reaction mixture flowing out of the third microchannel reactor followed by dissolving with a second organic solvent obtain a suspension; and transporting the suspension to the filter for filtration to collect a filtrate and a filter cake; wherein the filter cake is N-(2,6-dichlorophenyl)-2-hydroxyl-N-phenylacetamide (5);
(S6) concentrating the filtrate followed by dissolution with a third organic solvent and transportation to the third microchannel reactor for Smiles rearrangement to allow 2-(2,6 dichlorophenoxy)-N-phenylacetamide (4) in the filtrate to be converted into N-(2,6-dichlorophenyl)-2-hydroxyl-N-phenylacetamide (5); and repeating step (S5) to obtain another filter cake;
(S7) combining filter cakes followed by dissolving with a fourth organic solvent to obtain a N-(2,6-dichlorophenyl)-2-hydroxyl-N-phenylacetamide (5) solution; and transporting the N-(2,6-dichlorophenyl)-2-hydroxyl-N-phenylacetamide (5) solution to the second storage tank;
(S8) respectively transporting the N-(2,6-dichlorophenyl)-2-hydroxyl-N-phenylacetamide (5) solution in the second storage tank and a mixed solution of thionyl chloride and a second catalyst to the third mixer for mixing to obtain a third mixture; allowing the third mixture to flow out of the third mixer and enter the fourth microchannel reactor for chlorination;
(S9) subjecting a reaction mixture flowing out from the fourth microchannel reactor to continuous quenching, liquid-liquid extraction and separation to collect an organic phase;
(S10) concentrating the organic phase obtained in step (S9) followed by dissolution with a fifth organic solvent to obtain a N-(2,6-dichlorophenyl)-2-chloro-N-phenylacetamide (6) solution; transporting the N-(2,6-dichlorophenyl)-2-chloro-N-phenylacetamide (6) solution to the third storage tank;
(S11) respectively transporting the N-(2,6-dichlorophenyl)-2-chloro-N-phenylacetamide (6) solution and an organic solution of aluminum chloride to the fourth mixer for mixing and preheating to obtain a fourth mixture; allowing the fourth mixture to flow out of the fourth mixer and enter the fifth microchannel reactor for Friedel-Crafts alkylation;
(S12) subjecting a reaction mixture flowing out of the fifth microchannel reactor to continuous quenching, liquid-liquid extraction and separation to collect an organic phase;
(S13) concentrating the organic phase obtained in step (S12) followed by dissolving with a sixth organic solvent to obtain a 1-(2,6-dichlorophenyl)-1,3-dihydro-2H-indol-2-one (7) solution; transporting the 1-(2,6-dichlorophenyl)-1,3-dihydro-2H-indol-2-one (7) solution to the fourth storage tank;
(S14) respectively transporting the 1-(2,6-dichlorophenyl)-1,3-dihydro-2H-indol-2-one (7) solution in the fourth storage tank and a solution of an inorganic base to the fifth mixer for mixing to obtain a fifth mixture; and allowing the fifth mixture to flow out of the fifth mixer and enter the sixth microchannel reactor for hydrolysis to obtain the diclofenac sodium (1);
as shown in the following reaction scheme:
 |