| CPC A61M 16/0051 (2013.01) [A61M 16/024 (2017.08); A61M 16/104 (2013.01); A61M 16/12 (2013.01); G16H 20/10 (2018.01); G16H 50/20 (2018.01); A61M 16/0672 (2014.02); A61M 2016/0039 (2013.01); A61M 2202/0275 (2013.01); A61M 2205/505 (2013.01); A61M 2205/581 (2013.01); A61M 2205/583 (2013.01)] | 18 Claims |
|
1. A delivery system, comprising:
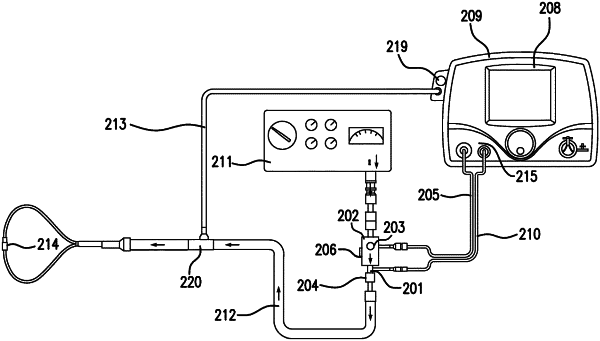
a control module comprising one or more processors and a processor readable memory, the control module configured to control a flow of therapeutic gas comprising nitric oxide that is mixed with a flow of breathing gas to form a gas mixture into a nebulizer device for delivery of the gas mixture to a patient;
a sample block configured to evaluate a presence and concentration of one or more of nitric oxide, oxygen and nitrogen dioxide in the gas mixture; and
a display configured to display an option to activate a nebulizer mode and receive run information including start nebulizer mode information and duration run nebulizer mode information,
wherein the control module is configured to:
store logic based information for the nebulizer mode, the start nebulizer mode information, and the duration run nebulizer mode information,
cause the sample block to cease evaluating the gas mixture in response to an input that the nebulizer device is in use for a duration of time based on the received duration run nebulizer mode information while continuing to deliver the gas mixture to the patient through the nebulizer device,
return to evaluating the gas mixture after the duration of time, and
cause to be presented on the display a calculated delivery dose of nitric oxide when the sample block is not evaluating the gas mixture and a measured concentration of nitric oxide when the sample block is evaluating.
|