| CPC A61K 9/2018 (2013.01) [A61K 9/0053 (2013.01); A61K 9/28 (2013.01); A61K 31/506 (2013.01)] | 12 Claims |
|
1. A method of treating a proliferative disorder in a patient in need thereof, comprising administering a therapeutically effective amount of a
a tablet for oral administration, comprising
dasatinib 1,2-propanediol solvate, dasatinib (R)-1,2-propanediol solvate, dasatinib (S)-1,2-propanediol solvate, or a combination thereof; and
at least one pharmaceutically acceptable excipient; and
a coating layer;
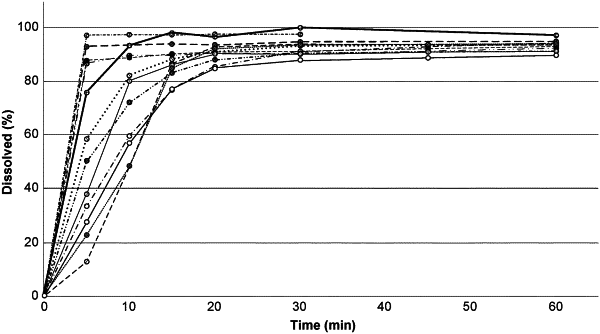
wherein the tablet releases at least 80% of the dasatinib within 20 minutes when the tablet is tested in a USP Type 2 in 500 mL of 0.01 M hydrochloric acid at about 37° C. and about 75 RPM.
|