| CPC A61K 47/6817 (2017.08) [A61K 47/6811 (2017.08)] | 5 Claims |
|
1. A method for producing an antibody-drug conjugate intermediate by addition of acid, wherein the reaction scheme of the method is:
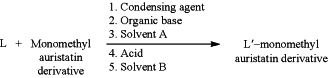 wherein
L is a linking group containing an acyl;
L′ is the residue of the linking group covalently attached to the monomethyl auristatin derivative;
solvent A and solvent B are polar or non-polar solvents; and the method comprises the following steps:
1) dissolving the linking group L, the condensing agent, and the organic base in the solvent A to obtain solution 1;
2) dissolving the monomethyl auristatin derivative and the acid in the solvent B to obtain solution 2; and
3) adding the solution 1 into the solution 2 to obtain the L′-monomethyl auristatin derivative via the condensation reaction between L and the monomethyl auristatin derivative;
wherein the molar amount of the organic base used in step 1) is greater than the molar amount of all free carboxyl group in the reaction system of step 3);
L is
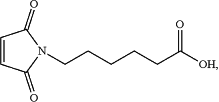 the monomethyl auristatin derivative is MMAF,
the condensing agent is HATU,
the organic base is one or more selected from N,N-diisopropylethylamine and triethylamine,
the solvent A and the solvent B are each independently selected from DMF, DMA and DMSO,
the acid is one or more selected from trifluoroacetic acid, p-toluenesulfonic acid, trifluoromethanesulfonic acid, (−)-10-camphorsulfonic acid, (+)-10-camphorsulfonic acid and methanesulfonic acid, and
the molar ratio of the acid to the monomethyl auristatin derivative is greater than 1.
|