| CPC A61K 47/64 (2017.08) [A61K 9/0019 (2013.01); A61K 9/0053 (2013.01); A61K 31/404 (2013.01); A61K 31/454 (2013.01); A61K 31/4545 (2013.01); A61K 38/45 (2013.01); A61K 47/545 (2017.08); A61K 47/556 (2017.08); A61P 35/02 (2018.01); C07D 209/24 (2013.01); C07D 471/04 (2013.01); C12Y 203/02 (2013.01)] | 19 Claims |
|
1. A compound comprising the structure of:
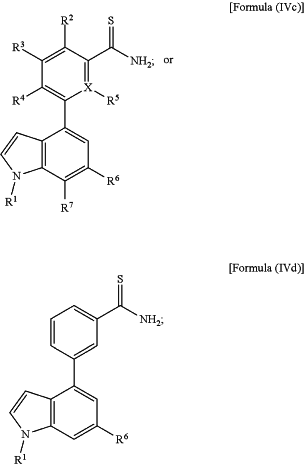 wherein X, when present, is CH or N;
wherein R1 is selected from H, alkyl, substituted alkyl, branched alkyl, a substituted brached alkyl, alkoxy, amine, substituted amine, thioalkyl, ketone, amide, a substituted amide, cyano, sulfonyl, carboxy, dialkylphosphine oxide, a carbocyclic ring, s sustituted carobocyclic ring, an aromatic ring, a substituted aromatic ring, a heterocyclic aromatic ring, a substituted heterocyclic aromatic ring, a substituted or non-substituted heterocyclic non-aromatic ring, carbocyclic or heterocyclic aromatic ring fused to another aromatic ring, a hydrogen bond donor, a hydrogen bond acceptor, and combinations thereof;
wherein R2, R3, R4, R5, and R7, when present, are independently selected from H, halogen, CH3, OH, SH, NH2, CN, CF3, CCl3, —CH2—CH3, —CH2—OH, —CH2NH2, —C(O)NH2, CH3SH, CH2Cl, CH2Br, CH2F, CHF2, CH2CN, CH2CF3, CH2Cl3, alkyl, haloalkyl, and alcohol; and
wherein R6 is selected from H, alkyl, substituted alkyl, hydroxy, alkoxy, amine, substituted amine, alkylamine, substituted alkylamine, thioalkyl, halogen, ketone, amide, substituted amide, alkylamide, substituted alkylamide, cyano, sulfonyl, carboxy, dialkylphosphine oxide, a carbocyclic ring, a substituted carbocyclic ring, an aromatic ring, a substituted aromatic ring, a heterocyclic aromatic ring, a substituted heterocyclic aromatic ring, a substituted or non-substituted heterocyclic non-aromatic ring, carbocyclic or heterocyclic aromatic ring fused to another aromatic ring, a hydrogen bond donor, a hydrogen bond acceptor, and combinations thereof;
or a salt thereof.
|