| CPC A61K 35/17 (2013.01) [A61K 39/0011 (2013.01); C12N 5/0636 (2013.01); A61K 2035/124 (2013.01); A61K 2039/5152 (2013.01); A61K 2039/5158 (2013.01); A61K 2039/545 (2013.01); A61K 2039/57 (2013.01); C12N 2501/23 (2013.01); C12N 2501/51 (2013.01); C12N 2501/515 (2013.01)] | 8 Claims |
|
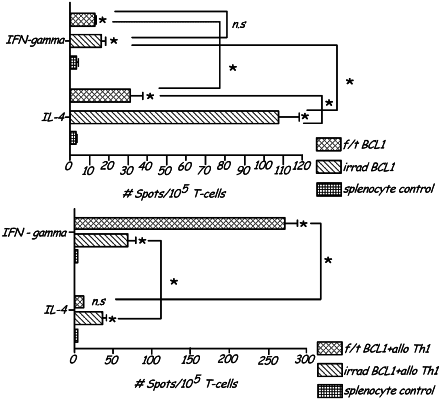
1. A method for treating an individual susceptible to one or more diseases comprising:
developing an anti-alloantigen immunity in an individual by administering a priming composition comprising allogeneic activated Th1 memory cells to the individual, wherein an individual's immune system increases the titer of the circulating anti-alloantigen specific Th1 cells, the priming composition administered prophylactically, prior to onset of the one or more diseases in the individual, wherein the one or more diseases is caused by a pathogen, the allogeneic Th1 memory cells activated by the cross-linking of CD3 and CD28 surface molecules at the time of formulation and administration, the priming composition comprising allogeneic Th1 memory cells, and cross-linking agents for cross-linking the CD3 and CD28 surface moieties on the Th1 memory cells, wherein the anti-alloantigen immunity is developed in the individual prior to administration of one or more disease-related antigens; and
administering an activating composition comprising allogeneic activated Th1 memory cells, and the one or more disease-related antigens to the primed individual, wherein the activating composition and the one or more disease-related antigens are administered at or after the onset of the one or more diseases in the individual, wherein the one or more diseases is suppressed by the increase in the titer of the circulating Th1 cells in the individual.
|