| CPC A61K 31/706 (2013.01) [A61K 31/502 (2013.01); A61K 45/06 (2013.01); A61P 3/10 (2018.01)] | 7 Claims |
|
1. A method of increasing cytoprotection in a cell type or tissue at risk of DNA damage in a subject, comprising administering to the subject in need thereof a therapeutically effective amount of a compound of Formula (V) or Formula (VI):
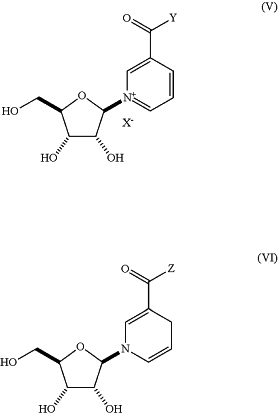 or pharmaceutically acceptable salts and a therapeutically effective amount of olaparib or pharmaceutically acceptable salts thereof, wherein:
Y and Z are —OH or —NH2;
X− is fluoride, chloride, bromide, iodide, nitrate, sulfate, sulfite, phosphate, bicarbonate, carbonate, thiocyanate, formate, acetate, trifluoroacetate, glycolate, lactate, gluconate, ascorbate, benzoate, oxalate, malonate, succinate, citrate, methanesulfonate, ethanesulfonate, propanesulfonate, benzenesulfonate, p-toluenesulfonate or trifluoromethanesulfonate; and the therapeutically effective amount of olaparib is not more than about 10% of the therapeutically effective amount of olaparib used in chemotherapy.
|