|
US 11,833,159 B2 |
|
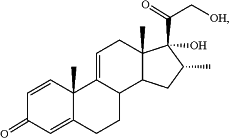
| Non-hormonal steroid modulators of NF-kB for treatment of disease |
| John M. McCall, Boca Grande, FL (US); Eric Hoffman, Rockville, MD (US); and Kanneboyina Nagaraju, Highland, MD (US) |
| Assigned to ReveraGen BioPharma, Inc., Rockville, MD (US) |
| Filed by ReveraGen BioPharma, Inc., Rockville, MD (US) |
| Filed on Oct. 28, 2020, as Appl. No. 17/082,521. |
| Application 15/483,863 is a division of application No. 15/229,947, filed on Aug. 5, 2016, granted, now 9,649,320, issued on May 16, 2017. |
| Application 14/164,779 is a division of application No. 13/678,253, filed on Nov. 15, 2012, granted, now 8,673,887, issued on Mar. 18, 2014. |
| Application 13/678,253 is a division of application No. 13/327,628, filed on Dec. 15, 2011, granted, now 8,334,279, issued on Dec. 18, 2012. |
| Application 13/327,628 is a division of application No. 12/473,921, filed on May 28, 2009, granted, now 8,207,151, issued on Jun. 26, 2012. |
| Application 17/082,521 is a continuation of application No. 16/226,061, filed on Dec. 19, 2018, granted, now 10,857,161. |
| Application 16/226,061 is a continuation of application No. 15/483,863, filed on Apr. 10, 2017, granted, now 10,206,933, issued on Feb. 19, 2019. |
| Application 15/229,947 is a continuation of application No. 14/164,779, filed on Jan. 27, 2014, granted, now 9,434,758, issued on Sep. 6, 2016. |
| Claims priority of provisional application 61/056,715, filed on May 28, 2008. |
| Prior Publication US 2021/0052606 A1, Feb. 25, 2021 |
| This patent is subject to a terminal disclaimer. |
| Int. Cl. A61K 31/573 (2006.01); A61K 31/575 (2006.01); C07J 5/00 (2006.01); A61K 31/57 (2006.01); A61P 1/04 (2006.01); A61P 1/16 (2006.01); A61P 3/00 (2006.01) |