| CPC A61K 31/53 (2013.01) [A61K 31/519 (2013.01); A61P 35/00 (2018.01); A61K 9/0053 (2013.01)] | 20 Claims |
|
1. A method of treating a myeloproliferative neoplasm or acute myeloid leukemia in a subject comprising administering to the subject a combination of a therapeutically effective amount of 2-methyl-1-[(4-[6-(trifluoromethyl)pyridin-2-yl]-6-{[2-(trifluoromethyl)pyridin-4-yl]amino}-1,3,5-triazin-2-yl)amino]propan-2-ol having the following formula:
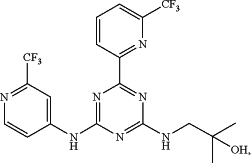 or a pharmaceutically acceptable salt, solvate, tautomer, stereoisomer, isotopologue, prodrug, metabolite, or a polymorph thereof (COMPOUND 1) and a therapeutically effective amount of a JAK2 inhibitor selected from TG101348, CYT387, lestaurtinib, AZD1480, pacritinib, XL019, NCB0-16562, NVP-BSK805, R723, hydroxycarbamide, SAR302503, tasocitinib and INCB16562, wherein the subject harbors a mutant allele of IDH2 and a mutant allele of JAK2.
|