| CPC A61K 31/454 (2013.01) [A61K 31/4545 (2013.01); A61K 31/498 (2013.01); A61P 35/00 (2018.01); C07D 401/04 (2013.01); C07D 401/14 (2013.01)] | 5 Claims |
|
1. A method of reducing the proliferation of a cell the method comprising, contacting the cell with a compound of Formula (I′)
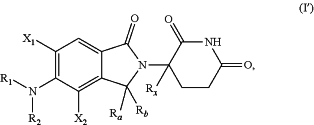 wherein:
X1 and X2 are each independently H, (C1-C4)alkyl, (C1-C6)alkoxy, (C1-C4)haloalkyl, (C1-C6)haloalkoxy, (C3-C7)cycloalkyl, halogen, CN, —OH, or —NH2;
Rx is H or D;
each Ra and Rb is independently H or D, or Ra and Rb together with the atom to which they are attached form ═(O);
R1 is
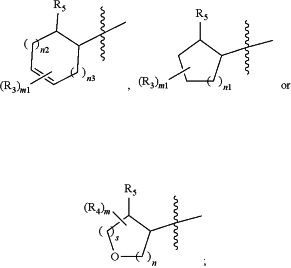 R2 is H, (C1-C6)alkyl, (C1-C6)haloalkyl, or (C3-C6)cycloalkyl; or
R2 and R7 together with the nitrogen atoms to which they are attached form a 6- or 7-membered heterocycloalkyl ring;
each R3 is independently at each occurrence (C1-C6)alkyl, (C1-C6)alkoxy, (C1-C6)haloalkyl, (C1-C6)haloalkoxy, halogen, CN, —OH, or —NH2; or
two R3 together with the carbon atoms to which they are attached form a (C3-C7)cycloalkyl or a 4- to 7-membered heterocycloalkyl ring consisting of 1-3 heteroatoms selected from O, N, and S; or two R3 together when on adjacent carbon atoms form a phenyl or a 5- or 6-membered heteroaryl ring consisting of 1-3 heteroatoms selected from O, N, and S; or
R3 and R7 together with the nitrogen and carbon atoms to which they are attached form a 5- or 6-membered heterocycloalkyl ring optionally consisting of 1 to 2 additional heteroatoms selected from O, N, and S, optionally substituted with one to four substituents each independently selected from (C1-C6)alkyl, (C1-C6)haloalkyl, halogen, —OH, CN, and —NH2;
each R4 is (C1-C6)alkyl, (C1-C6)alkoxy, (C1-C6)haloalkyl, (C1-C6)haloalkoxy, halogen, —OH, or —NH2;
R5 is —OR5 or —NR7R7;
R6 is H, (C1-C6)alkyl, (C1-C6)haloalkyl, —C(O)(C1-C6)alkyl, (C3-C7)cycloalkyl, 5- or 6-membered heterocycloalkyl consisting of 1-3 heteroatoms selected from O, N, and S, (C6-C10)aryl, or 5- or 6-membered heteroaryl consisting of 1-3 heteroatoms selected from O, N, and S, wherein the alkyl is optionally substituted with one to three substituents each independently selected from (C6-C10)aryl and 5- or 6-membered heteroaryl consisting of 1-3 heteroatoms selected from O, N, and S;
R7 and R7′ are each independently H, (C1-C6)alkyl, (C1-C6)haloalkyl, (C3-C7)cycloalkyl, 5- or 6-membered heterocycloalkyl consisting of 1-3 heteroatoms selected from O, N, and S, (C6-C10)aryl, or 5- or 6-membered heteroaryl consisting of 1-3 heteroatoms selected from O, N, and S, wherein the alkyl is optionally substituted with one to three R8 and wherein the cycloalkyl, heterocycloalkyl, aryl, and heteroaryl are optionally substituted with one to four R11; or
R7 and R7′ together with the nitrogen atom to which they are attached form a 4- to 7-membered heterocycloalkyl ring optionally consisting of 1 to 2 additional heteroatoms selected from O, N, and S, optionally substituted with one to four R9; or
R2 and R7 together with the nitrogen atoms to which they are attached form a 6- or 7-membered heterocycloalkyl ring; or
R3 and R7 together with the nitrogen and carbon atoms to which they are attached form a 5- or 6-membered heterocycloalkyl ring optionally consisting of 1 to 2 additional heteroatoms selected from O, N, and S, optionally substituted with one to four substituents each independently selected from (C1-C6)alkyl, (C1-C6)haloalkyl, halogen, —OH, CN, and —NH2;
each R8 is —C(O)OH, (C3-C7)cycloalkyl, 4- to 7-membered heterocycloalkyl ring consisting of 1-3 heteroatoms selected from O, N, and S, (C6-C10)aryl, or 5- or 6-membered heteroaryl consisting of 1-3 heteroatoms selected from O, N, and S, wherein the cycloalkyl, heterocycloalkyl, aryl, and heteroaryl are optionally substituted with one to four R10;
each R9 is independently at each occurrence (C1-C6)alkyl, (C1-C6)alkoxy, (C1—C6)haloalkyl, (C1-C6)haloalkoxy, halogen, —OH, CN, —NR12R13, or —NH2, wherein the alkoxy is optionally substituted with one to three substituents independently selected from (C3-C7)cycloalkyl, 4- to 7-membered heterocycloalkyl ring consisting of 1-3 heteroatoms selected from O, N, and S, (C6-C10)aryl, and 5- or 6-membered heteroaryl consisting of 1-3 heteroatoms selected from O, N, and S; or
two R9 together with the atoms to which they are attached form a (C5-C7)cycloalkyl or a 5- to 7-membered heterocycloalkyl ring consisting of 1-2 heteroatoms selected from O, N, and S;
each R10 is independently at each occurrence (C1-C6)alkyl, (C1-C6)haloalkyl, (C1-C6)alkoxy, (C1-C6)haloalkoxy, halogen, —OH, CN, or —NH2; or
two R10 together with the atoms to which they are attached form a (C4-C7)cycloalkyl or a 5- to 7-membered heterocycloalkyl ring consisting of 1-2 heteroatoms selected from O, N, and S optionally substituted with one or more (C1-C6)alkyl, (C1-C6)haloalkyl, halogen, —OH, CN, or —NH2;
each R11 is independently at each occurrence (C1-C6)alkyl, (C1-C6)haloalkyl, (C1-C6)alkoxy, (C1-C6)haloalkoxy, halogen, —OH, CN, or —NH2;
R12 and R13 are each independently selected from (C1-C6)alkyl, (C1-C6)haloalkyl, (C3-C7)cycloalkyl, 4- to 7-membered heterocycloalkyl ring consisting of 1-3 heteroatoms selected from O, N, and S, (C6-C10)aryl, and 5- or 6-membered heteroaryl consisting of 1-3 heteroatoms selected from O, N, and S;
m and m1 are each independently 0, 1 or 2;
n1 is 0, 1, 2, or 3;
n2 and n3 are each independently 1 or 2; and
each s and n is independently 1, 2, or 3, wherein s+n is ≤4;
or a pharmaceutically acceptable salt, stereoisomer, or tautomer thereof and reducing IKZF2 protein levels.
|