| CPC A61K 31/18 (2013.01) [A61K 31/16 (2013.01); A61K 45/06 (2013.01)] | 21 Claims |
|
1. A method of treating cancer in a subject in need thereof, the method comprising administering to cancer cells of the subject a therapeutically effective amount of a compound having the formula:
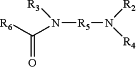 wherein R2 is selected from the group consisting of hydrogen, substituted or unsubstituted C1-C24 alkyl, C2-C24 alkenyl, C2-C24 alkynyl, C3-C20 aryl, heterocycloalkenyl containing from 5-6 ring atoms, heteroaryl or heterocyclyl containing from 5-14 ring atoms, C6-C24 alkaryl, C6-C24 aralkyl, halo, silyl, hydroxyl, sulfhydryl, C1-C24 alkoxy, C2-C24 alkenyloxy, C2-C24 alkynyloxy, C5-C20 aryloxy, acyl, acyloxy (—O-acyl), C2-C24 alkoxycarbonyl (—(CO)—O-alkyl), C6-C20 aryloxycarbonyl (—(CO)—O-aryl), C2-C24 alkylcarbonato (—O—(CO)—O-alkyl), C6-C20 arylcarbonato (—O—(CO)—O-aryl), carboxy (—COOH), carboxylato (—COO−), carbamoyl (—(CO)—NH2), C1-C24 alkyl-carbamoyl (—(CO)—NH(C1-C24 alkyl)), arylcarbamoyl (—(CO)—NH-aryl), thiocarbamoyl (—(CS)—NH2), carbamido (—NH—(CO)—NH2), cyano (—CN), isocyano (—N+C−), cyanato (—O—CN), isocyanato (—O—N+═C−), isothiocyanato (—S—CN), azido (—N═N+═N−), formyl (—(CO)—H), thioformyl (—(CS)—H), amino (—NH2), C1-C24 alkyl amino, C5-C20 aryl amino, C2-C24 alkylamido (—NH—(CO)-alkyl), C6-C20 arylamido (—NH—(CO)-aryl), sulfanamido, imino, alkylimino, arylimino, nitro (—NO2), nitroso (—NO), sulfo (—SO2—OH), sulfonato (—SO2—O−), C1-C24 alkylsulfanyl, arylsulfanyl, C1-C24 alkylsulfinyl (—(SO)-alkyl), C5-C20 arylsulfinyl (—(SO)-aryl), C1-C24 alkylsulfonyl (—SO2-alkyl), C5-C20 arylsulfonyl (—SO2-aryl), sulfonamide, phosphono (—P(O)(OH)2), phosphonato (—P(O)(O−)2), phosphinato (—P(O)(O−)), phospho (—PO2), phosphino (—PH2), polyalkyl ethers (—[(CH2)nO]m), phosphates, and phosphate esters;
R3 and R4 are the same or different and selected from the group consisting of hydrogen and substituted or unsubsubstituted C1-C6 alkyl;
R5 is selected from the group consisting of a substituted or unsubstituted C2-C6 alkylene, C2-C24 alkenylene, C2-C24 alkynylene, C3-C20 arylene, heterocycloalkenylene containing from 5-6 ring atoms, heteroarylene or heterocyclylene containing from 5-14 ring atoms, C6-C24 alkarylene, and C6-C24 aralkylene;
wherein R6 is selected from the group consisting of substituted or unsubstituted C1-C24 alkyl, C2-C24 alkenyl, C2-C24 alkynyl, C3-C20 aryl, heterocycloalkenyl containing from 5-6 ring atoms, heteroaryl or heterocyclyl containing from 5-14 ring atoms, C6-C24 alkaryl, C6-C24 aralkyl, C1-C24 alkoxy, C2-C24 alkenyloxy, C2-C24 alkynyloxy, C5-C20 aryloxy, acyl, acyloxy (—O-acyl), C2-C24 alkoxycarbonyl (—(CO)—O-alkyl), C6-C20 aryloxycarbonyl (—(CO)—O-aryl), C2-C24 alkylcarbonato (—O—(CO)—O-alkyl), C6-C20 arylcarbonato (—O—(CO)—O-aryl), carboxy (—COOH), carboxylato (—COO−), carbamoyl ((CO)—NH2), C1-C24 alkyl-carbamoyl (—(CO)—NH(C1-C24 alkyl)), and arylcarbamoyl (CO)—NH-aryl);
R6 is not a methyl group if R5 is 1,6-hexylene, R2 is an acetyl group, and R3 and R4 are hydrogen; and pharmaceutically acceptable salts thereof.
|