| CPC A61B 17/0057 (2013.01) [A61B 2017/00004 (2013.01); A61B 2017/00526 (2013.01); A61B 2017/00606 (2013.01); A61B 2017/00623 (2013.01); A61B 2017/00867 (2013.01); A61B 2017/00884 (2013.01); A61B 2017/3425 (2013.01); A61B 2090/3966 (2016.02)] | 20 Claims |
|
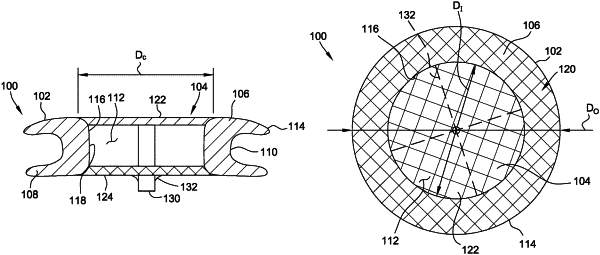
1. An occlusive medical device comprising:
a frame comprising:
a distal annular flange having a radially outer surface and a radially inner surface;
a proximal annular flange having a radially outer surface and a radially inner surface; and
a waist member extending between and connecting the distal annular flange to the proximal annular flange,
wherein the radially inner surface of the distal annular flange, the waist member, and the radially inner surface of the proximal annular flange define an unobstructed passageway through the frame, wherein an unobstructed area of the distal annular flange delimiting the passageway and defined by the radially inner surface of the distal annular flange represents between 50% and 70% of a total surface area defined by the radially outer surface of the distal annular flange, and wherein an unobstructed area of the proximal annular flange delimiting the passageway and defined by the radially inner surface of the proximal annular flange represents between 50% and 70% of a total surface area defined by the radially outer surface of the proximal annular flange; and
at least one closure coupled to the frame and configured to close the passageway to: (i) provide an occlusive effect, and (ii) enable subsequent access through the passageway when the occlusive medical device is deployed at a target site, wherein the at least one closure is formed from a woven, knitted, or braided material.
|