| CPC A61K 31/551 (2013.01) [A61K 31/165 (2013.01); A61K 31/167 (2013.01); A61K 31/277 (2013.01); A61K 31/341 (2013.01); A61K 31/351 (2013.01); A61K 31/402 (2013.01); A61K 31/415 (2013.01); A61K 31/4196 (2013.01); A61K 31/42 (2013.01); A61K 31/429 (2013.01); A61K 31/4245 (2013.01); A61K 31/437 (2013.01); A61K 31/4985 (2013.01); A61K 31/506 (2013.01); A61K 31/5025 (2013.01); A61K 31/519 (2013.01)] | 16 Claims |
|
1. A method of treating a disease or disorder in a subject, wherein the disease or disorder is selected from the group consisting of a renal, hepatic and/or bowel ischemia, heart failure, cardiomyopathy, pancreatic cancer, gastrointestinal cancer, breast cancer, cervical carcinoma, liver cancer, and leukemia, the method comprising administering to the subject a compound of formula (III) in an amount effective to activate mitofusin 1 or mitofusin 2 to treat the disease or disorder,
wherein
formula (III) is
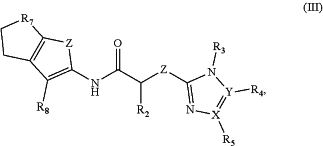 wherein
R2 is H, or C1-C4 alkyl;
R3 and R5 are independently H, alkenyl, alkynyl, cycloalkyl, CHCH3N(CH3)2, heterocycloalkyl, benzyl, aryl, heteroaryl, aralkyl, heteroaralkyl, or optionally substituted aryl, aralkyl, heteroaryl, or heteroaralkyl, wherein the optional substituent is one or more of F, Cl, Br, I, OH, CH3, SH, or CF3;
R4 is H, alkenyl, alkynyl, cycloalkyl, CHCH3N(CH3)2, heterocycloalkyl, benzyl, aryl, 5- or 6-membered heteroaryl, aralkyl, heteroaralkyl, or optionally substituted 6-membered aryl, aralkyl, 5- or 6-membered heteroaryl, or heteroaralkyl, wherein the optional substituent is one or more of F, Cl, Br, I, OH, CH3, SH or CF3;
R7 is CH2, CH2CH2, CH2CH2CH2 or CH2CH(CH3);
R8 is CN, CONH2, COOH or COOR9;
R9 is C1-C4 alkyl;
X and Y are independently N or C;
Z is S;
provided that in Formula III when R4 or R5 is heteroaryl and R8 is CONH2, R2 is not H;
or a pharmaceutically acceptable salt, ester or prodrug thereof, and
provided that the compound is not any of the following:
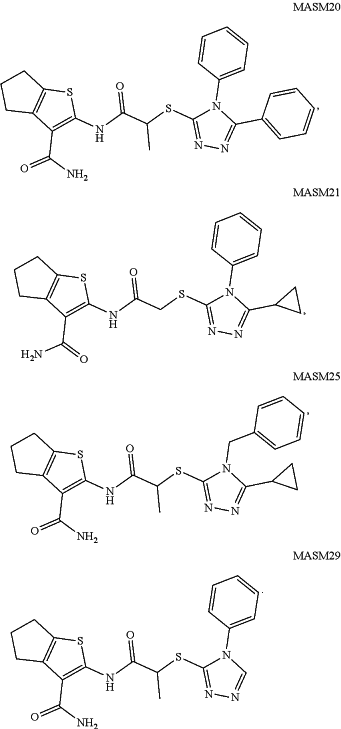 |