| CPC A61K 31/4745 (2013.01) [A61K 31/473 (2013.01); A61P 25/14 (2018.01); A61P 25/28 (2018.01); C07D 471/04 (2013.01)] | 7 Claims |
|
1. A process for preparing a deutetrabenazine drug product in the form of a tablet, that has no more than 0.4 area-% of Compound 2 relative to the concentration of deutetrabenazine in the drug product after storage at room temperature for one month, comprising:
obtaining a deutetrabenazine drug substance comprising Compound 2 in an amount that is not more than 0.15 area-%, relative to the concentration of the deutetrabenazine in the deutetrabenazine drug substance;
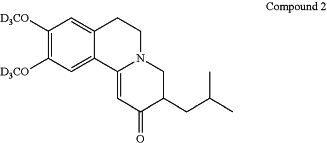 wherein the amount of Compound 2 is determined by an HPLC method comprising a C18, 150×4.6 mm, 3.5 μm column and a photodiode array/ultraviolet detector at 220 nm; and
admixing the deutetrabenazine drug substance with an excipient to produce the deutetrabenazine drug product in the form of a tablet;
wherein the deutetrabenazine drug product has no more than 0.4 area-% of Compound 2, relative to the concentration of deutetrabenazine in the drug product, based on a determination by the HPLC method, after the storage at room temperature for one month.
|