| CPC G01N 33/6845 (2013.01) [G01N 33/542 (2013.01); G01N 33/54353 (2013.01); G01N 2440/00 (2013.01)] | 6 Claims |
|
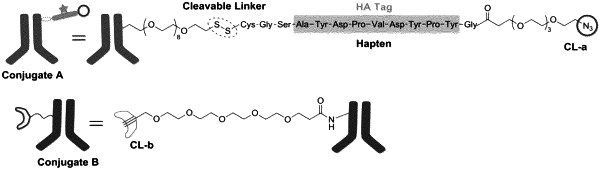
1. A composition comprising a first modified binding moiety, wherein the first modified binding moiety comprises:
(a) at least one a cleavable bridge component; and
(b) a first antibody is covalently conjugated to the at least one cleavable bridge component;
wherein the cleavable bridge component has the covalently conjugated structure:
—[cleavage site]—[detectable moiety]—[chemical ligation group], wherein the cleavage site is more proximal to the first antibody than is the detectable moiety and the chemical ligation group, wherein the chemical ligation group is at or near a terminus of the cleavable bridge component, and wherein the chemical ligation group comprises a first reactive functional group; wherein the cleavage site is selected from the group consisting of a disulfide bond, a vicinal diol, 1,4-Bis(2-hydroxymethyl)benzene (“1,4-BHMB”), 1,4-Bis(2-hydroxethyl)benzene (“1,4-BHEB”), 1,3-Bis(2-hydroxymethyl)benzene (“1,3-BHMB”), 2,5-bi s(hydroxymethyl)furan (“2,5-BHMF”), and a vicinal hydroxylamine; wherein the detectable moiety is selected from the group consisting of a peptide tag and an oligonucleotide; and wherein the first reactive functional group is selected from the group consisting of:
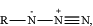 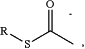 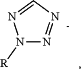 RC≡CH,
RC═CH2,
a halogen, and
a pyridine including one R group,
wherein R is H.
|