| CPC C09D 5/032 (2013.01) [B05D 1/06 (2013.01); C08G 63/183 (2013.01); C08G 63/60 (2013.01); C08K 5/0025 (2013.01); C08K 5/20 (2013.01); C09D 5/035 (2013.01); C09D 167/02 (2013.01); C08G 2150/20 (2013.01)] | 17 Claims |
|
1. An one-component (1K) thermosetting powder coating composition A (PCC A) comprising:
a) a binder (B) in an amount of at least 30 and at most 100 pph PCC A, wherein the B consists of:
i) a polyester binder (PB) in an amount of at least 90.0 and at most 98.0 pph B, wherein the PB consists of a polyester resin A (PRA) and a polyester resin B (PRB), and wherein the weight ratio of the weight amount of PRA to the weight amount of PRB (R=weight of PRA/weight of PRB) is at least 0.50 and at most 4.00, and wherein the difference between the acid value of the PRB (AVB) and the acid value of the PRA (AVA) (DeltaAV=AVB−AVA) is at least 20 and at most 59 mg KOH/g, and
ii) a crosslinker in an amount of at least 2.0 and at most 10.0 pph B, wherein the crosslinker is selected from the group consisting of β-hydroxylalkyl-amides and mixtures thereof, wherein the crosslinker is able to react with the PB, and
b) a pigment in an amount of at least 0 and at most 70 pph PCC A,
and
wherein the PRA is the polycondensation reaction product of at least:
a component A1 in an amount of at least 45.0 and at most 49.9, mol % based on PRA, wherein the component A1 consists of: i) 2,2-dimethylpropane-1,3-diol in an amount of at least 70 and at most 100% of the total amount of moles making up the A1, and ii) a diol-A1 selected from the group consisting of C2-18 excluding the 2,2-dimethylpropane-1,3-diol, and mixtures thereof, in an amount of at least 0 and at most 30% of the total amount of moles making up the A1,
and
a component A2 in an amount of at least 48.0 and at most 55.0, mol % based on PRA, wherein the component A2 consists of: i) a dicarboxylic-acid-A2a selected from the group consisting of terephthalic acid, esters of terephthalic acid), isophthalic acid, esters of isophthalic acid, and mixtures thereof, in an amount of at least 90 and at most 100% of the total amount of moles making up the A2, and ii) a dicarboxylic-acid-A2b selected from the group consisting of C6-18 aliphatic saturated dicarboxylic acids, esters of C6-18 aliphatic saturated dicarboxylic acids, hexahydrophthalic anhydride, and mixtures thereof, in an amount of at least 0 and at most 10% of the total amount of moles making up the A2,
and
a component A3 in an amount of at least 0 and at most 2.7 mol % based on PRA, wherein the component A3 is selected from the group consisting of: i) an alcohol-A3 selected from the group consisting of C3-10 aliphatic saturated alcohols having at least 3 and at most 6 hydroxyl groups, and mixtures thereof, and ii) a carboxylic-acid-anhydride-A3 selected from the group consisting of trimellitic acid anhydride, pyromellitic acid anhydride, and mixtures thereof,
wherein the total amount of monomers reacted to produce the PRA is 100 mol %, and wherein the PRA
is carboxylic acid functional having an acid value (AVA) as measured titrimetrically by ISO 2114-2000, of at least 15 and at most 35, and a hydroxyl value (OHVA) as measured titrimetrically by ISO 4629-2-2016, of at most 10 mg KOH/g,
has a glass transition temperature (TgA) as determined by Differential Scanning Calorimetry (DSC) according to the description, of at least 40 and at most 75° C., and
has a functionality (fA) of at least 2.0 and at most 3.5 and
has a number average molecular weight (MnA) as determined by Size Exclusion Chromatography (SEC) according to the description, of at least 1100 and at most 10000 Da,
has a weight average molecular weight (MwA) as determined by SEC according to the description, of at least 2200 and at most 20000 Da,
has a polydispersity DA (=MwA/MnA) of at least 2.0 and at most 4.0, and
has a melt viscosity (NA) as determined via rheometry according to the description, of at least 15 and at most 150 Pa·s,
and
wherein the PRB is the polycondensation reaction product of at least:
a component B1 in an amount of at least 42 and at most 49.9, mol % based on PRB, wherein the component B1 consists of: i) a diol-B1a selected from the group consisting of ethylene glycol, 1,2-propane diol, 1,3-propane diol, and mixtures thereof, in an amount of at least 50 and at most 90% of the total amount of moles making up the B1, and ii) a diol-B1b selected from the group consisting of C4-18 and mixtures thereof, in an amount of at least 10 and at most 50% of the total amount of moles making up the B1,
and
a component B2, in an amount of at least 42 and at most 55, mol % on PRB, wherein the component B2 consists of: i) a dicarboxylic-acid-B2a selected from the group consisting of terephthalic acid, esters of terephthalic acid, isophthalic acid, esters of isophthalic acid, and mixtures thereof, in an amount of at least 80 and at most 100% of the total amount of moles making up the B2, and ii) a dicarboxylic-acid-B2b selected from the group consisting of C6-18 aliphatic saturated dicarboxylic acids, esters of C6-18 aliphatic saturated dicarboxylic acids, hexahydrophthalic anhydride, and mixtures thereof, in an amount of at least 0 and at most 20% of the total amount of moles making up the B2,
and
a component B3 in an amount of at least 0.5 and at most 8.5, mol % on PRB, wherein the component B3 is selected from the group consisting of: i) an alcohol-B3 selected from the group consisting of C3-10 aliphatic saturated alcohols having at least 3 and at most 6 hydroxyl groups, and mixtures thereof, and ii) a carboxylic-acid-anhydride-B3 selected from the group consisting of trimellitic acid anhydride, pyromellitic acid anhydride, and mixtures thereof,
and wherein the total amount of monomers reacted to produce the PRB is 100 mol %, and wherein the PRB
is a branched carboxylic acid functional having an acid value (AVB) as measured titrimetrically by ISO 2114-2000, of at least 40 and at most 74,
and a hydroxyl value (OHVA) as measured titrimetrically by ISO 4629-2-2016, of at most 10 mg KOH/g,
has a glass transition temperature (TgB) as determined by DSC according to the description, of at least 40 and at most 80° C., and
has a functionality (fB) of at least 2.7 and at most 6.0, and
has a number average molecular weight (MnB) as determined by SEC according to the description, of at least 1100 and at most 10000 Da,
has a weight average molecular weight (MwB) as determined by SEC according to the description, of at least 3000 and at most 25000 Da,
has a polydispersity DB (=MwB/MnB) of at least 2.5 and at most 4.0, and
has a melt viscosity (NB) as determined via rheometry according to the description, of at least 8 and at most 120 Pa·s,
and
wherein the β-hydroxylalkyl-amide compounds are chemical compounds having the average chemical structure represented by the following Formulae 1 or 2:
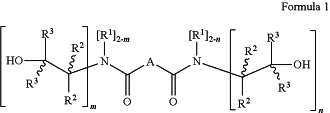 wherein:
n ranges from and including 1 up to and including 2;
m ranges from and including 1 up to and including 2;
A is a C1-60 optionally-substituted-hydrocarbylene linking group;
R1 is hydrogen, or a C1-5 alkyl group optionally substituted with one or more hydroxyl groups;
R2 is hydrogen, or a C1-5 alkyl group;
R3 is hydrogen, or a C1-5 alkyl group;
while one of the groups R2 and one of the groups R3 of the unit m, may also form—together with the adjacent carbon atoms—, a cycloalkyl group; and/or while one of the groups R2 and one of the groups R3 of the unit n, may also form—together with the adjacent carbon atoms—, a cycloalkyl group,
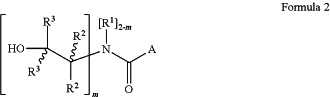 wherein:
m ranges from and including 1 up to and including 2;
A is a C1-60 optionally-substituted-hydrocarbyl;
R1 is hydrogen, or a C1-5 alkyl group optionally substituted with one or more hydroxyl groups;
R2 is hydrogen, or a C1-5 alkyl group;
R3 is hydrogen, or a C1-5 alkyl group;
while one of the groups R2 and one of the groups R3, may also form—together with the adjacent carbon atoms—, a cycloalkyl group.
|