| CPC C07H 19/23 (2013.01) [A61K 45/06 (2013.01)] | 19 Claims |
|
1. A method of treating cancer in a subject, comprising administering to the subject a compound of Formula (IV), or a pharmaceutically acceptable salt thereof:
wherein:
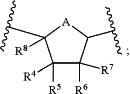
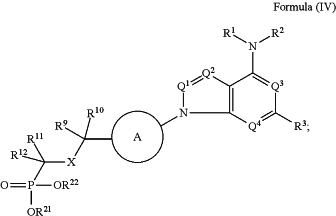 Ring A is
 Q2 is N or CW;
Q3 is N and Q4 is CW;
or Q3 is CW and Q4 is N;
each W is independently hydrogen, halogen, —CN, —ORb, NRcRd, C1-C6 alkyl, or C1-C6 haloalkyl;
A is —O— or —CH2—;
X is —O—, —S—, —S(═O)2—, —NR17—, or —C(R18)2—;
R1 and R2 are independently hydrogen, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6 alkyl(aryl), C1-C6 alkyl(heteroaryl), C1-C6 alkyl(cycloalkyl), or C1-C6 alkyl(heterocycloalkyl); wherein each alkyl, Amyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently, optionally substituted with one, two, or three R1a;
or R1 and R2 are taken together with the nitrogen atom to which they are attached to form a heterocycloalkyl optionally substituted with one, two, or three R1b;
each R1a and R1b is independently oxo (when feasible), halogen, —CN, —ORb, —SRb, —S(═O)Ra, —NO2, —NRcRd, —S(═O)2Ra, —NRbS(═O)2Ra, —S(═O)2NRcRd, —C(═O)ORb, —OC(═O)ORb; —C(═O)NRcRd, —OC(═O)NRcRd, —NRbC(═O)NRcRd, —NRbC(═O)Ra, —NRbC(═O)ORb, C1-C6 alkyl, C1-C6 fluoroalkyl, C2-C6 alkenyl, C2-C6 alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6 alkyl(aryl), C1-C6 alkyl(heteroaryl), C1-C6 alkyl(cycloalkyl), or C1-C6 alkyl(heterocycloalkyl);
R3 is hydrogen, halogen, —CN, —ORb, —SRb, —NRcRd, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C1-C6 haloalkyl, C1-C6 hydroxyalkyl, C1-C6 heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6 alkyl(aryl), C1-C6 alkyl(heteroaryl), C1-C6 alkyl(cycloalkyl), or C1-C6 alkyl(heterocycloalkyl); wherein each alkyl; alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one, two; or three R3a;
each R3a is independently oxo (when feasible), halogen, —CN, —ORb, —NRcRd, —C(═O)Ra, —C(═O)ORb, —C(═O)NRcRd, C1-C6 alkyl, C1-C6 fluoroalkyl, C2-C6 alkenyl, C2-C6 alkynyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl,
R4 and R7 are independently hydrogen, halogen, —ORb, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 alkynyl, cycloalkyl, heterocycloalkyl, aryl; or heteroaryl;
R5 and R6 are independently hydrogen, halogen, —ORb, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 alkynyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
R8 is hydrogen, halogen, —ORb, —NRcRd, C1-C6 alkyl, or C1-C6 haloalkyl;
R9 and R10 are independently hydrogen, halogen, C1-C6 alkyl, or C1-C6 haloalkyl;
R11 is hydrogen, halogen, —CN, —OR13, —SR13, —S(═O)R14, —NO2, —NR15R16, —S(═O)2R14, —NR13S(═O)2R14, —S(═O)2NR15R16, —C(═O)R14, —OC(═O)R14, —C(═O)OR13, —OC(═O)OR13, NR13C(═O)OR13, C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 hydroxyalkyl, C2-C6 alkenyl, C2-C6 alkynyl, C1-C6 heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl, wherein each alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one, two, or three R11a;
R12 is halogen, —CN, —OR13, —SR13, —S(═O)R14, —NO2, —NR15R16, —S(═O)2R14, —NR13S(═)2R14, —S(═)2NR15R16, —C(═O)R14, —OC(═O)R14, —C(═O)OR13, —OC(═O)OR13, —C(═O)NR15R16, —OC(═O)NR15R16, —NR13C(═O)NR15R16, —NR13C(═O)R14, —NR13C(═O)OR13, C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 hydroxyalkyl, C2-C6 alkenyl, C2-C6 alkynyl, C1-C6 heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; wherein each alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one, two, or three R12a;
or R11 and R12 are taken together to form a cycloalkyl or heterocycloalkyl each optionally substituted with one, two, or three R11b;
each R11a, R11b, and R12a is independently oxo (when feasible), halogen, —CN, —OR13, —SR13, —S(═O)R14, —NO2, —NR15R16, —S(═O)2R14, —NR13S(═O)2R14, —S(═O)2NR15R16, —C(═O)R14, —OC(═O)R14, —C(═O)OR13, —OC(═O)OR13, —C(═O)NR15R16, —OC(═O)NR15R16, —NR13C(═O)NR15R16, —NR13C(═O)R14, —NR13C(═O)OR13, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 alkynyl, C1-C6 hydroxyalkyl, C1-C6 heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; wherein each alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one, two, or three R11c;
each R11c is independently oxo (when feasible), halogen, —CN, —ORb, —SRb, —S(═O)Ra, —NO2, —NRcRd, —S(═)2Ra, —NRbS(═O)2Ra, —S(═O)2NRcRd, —C(═O)Ra, —OC(═O)Ra, —C(═O)ORb, —OC(═O)ORb, —C(═O)NRcRd, —OC(═O)NRcRd, —NRbC(═O)NRcRd, —NRbC(═O)Ra, —NRbC(═O)ORb, C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 hydroxyalkyl, C2-C6 alkenyl, C2-C6 alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6 alkyl(aryl), C1-C6 alkyl(heteroaryl), C1-C6 alkyl(cycloalkyl), or C1-C6 alkyl(heterocycloalkyl);
each R13 is independently hydrogen, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, alkynyl, C1-C6 heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; wherein each alkyl alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one, two, or three R13a;
each R14 is independently C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 alkynyl, C1-C6 heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; wherein each alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one, two, or three R14a;
each R15 and R16 is independently hydrogen, C1-C6 alkyl; C1-C6 haloalkyl, alkenyl, C2-C6 alkynyl, C1-C6 heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; wherein each alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one, two, or three R15a;
or R15 and R16 are taken together with the nitrogen atom to which they are attached to form a heterocycloalkyl optionally substituted with one, two, or three R15b;
each R13a, R14a, R15a, and R15b is independently oxo (when feasible), halogen, —CN, —ORb, —SRb, —S(═O)Ra, —NO2, —NRcRd, —S(═O)2Ra, —NHS(═O)2Ra, —S(═O)2NRcRd, —C(═O)Ra, —OC(═O)Ra, —C(═O)ORb, —OC(═O)ORb, —C(═O)NRcRd, —OC(50 O)NRcRd, —NRbC(═O)NRcRd, —NRbC(═O)Ra, —NRbC(═O)ORb, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl; C1-C6 alkyl(aryl), C1-C6 alkyl(heteroaryl), C1-C6 alkyl(cycloalkyl), or C1-C6 alkyl(heterocycloalkyl); wherein each alkyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one, two, or three oxo (when feasible), halogen, —CN, —ORb, —S(═O)Ra, —NO2, —NRcRd; —S(═O)2Ra, —S(═O)2NRcRd, —C(═O)Ra, —C(═O)ORb, —C(═O)NRcRd, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, or C2-C6 alkynyl;
R17 is hydrogen, —CN, —ORb, —S(═O)2Ra, —C(═O)Ra, —C(═O)ORb; —C(═O)NRa, C1-C6 alkyl, C1-C6 fluoroalkyl, C2-C6 alkenyl, C2-C6 alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6 alkyl(aryl), C1-C6 alkyl(heteroaryl), C1-C6 alkyl(cycloalkyl), or C1-C6 alkyl(heterocycloalkyl);
each R18 is independently hydrogen, halogen, —CN, —ORb, —SRb, —S(═O)Ra, —NO2, —NRcRd, —S(═O)2Ra, —NHS(═O)2Ra, —S(═O)2NRcRd, —C(═O)Ra, —OC(═O)Ra, —C(═O)ORb, —OC(═O)ORb, —C(═O)NRcRd, —OC(═O)NRcRd, —NRbC(═O)NRcRd, —NRbC(═O)Ra, —NRbC(═O)ORb, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6 alkyl(aryl), C1-C6 alkyl(heteroaryl), C1-C6 alkyl(cycloalkyl), or C1-C6 alkyl(heterocycloalkyl);
R21 and R22 are independently hydrogen, C1-C20 alkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6 alkyl(aryl), C1-C6 alkyl(heteroaryl), C1-C6 alkyl(cycloalkyl), C1-C6 alkyl(heterocycloalkyl); wherein each alkyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one, two, or three R21a;
or R21 and R22 are taken together with the atoms to which they are attached to form a heterocycloalkyl optionally substituted with one, two, or three R21b;
each R21a and R21b is independently oxo (when feasible), halogen, —CN, —ORb, —SRb, —S(═O)Ra, —NO2, —NRcRd, —S(═O)2Ra, —NHS(═O)2Ra, —S(═O)2NRcRd, —C(═O)Ra, —OC(═O)Ra, —C(═O)ORb, —OC(═O)ORb, —C(═O)NRcRd, —OC(═O)NRcRd, —NRbC(═O)NRcRd, —NRbC(═O)Ra, —NRbC(═O)ORb, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkyl(aryl), C1-C6 alkyl(heteroaryl), C1-C6 alkyl(cycloalkyl), or C1-C6 alkyl(heterocycloalkyl);
each Ra is independently C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C1-C6 heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; wherein the alkyl, alkenyl, alkynyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one, two, or three oxo (when feasible), halogen, —OH, C1-C6 alkyl, or C1-C6 haloalkyl;
each Rb is independently hydrogen, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C1-C6 heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; wherein the alkyl, alkenyl, alkynyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one, two, or three oxo (when feasible), halogen, —OH, C1-C6 alkyl, or C1-C6 haloalkyl; and
each Rc and Rd is independently hydrogen, C1-C6 alkyl, C2-C6 alkenyl, alkynyl, C1-C6 heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; wherein the alkyl, alkenyl, alkynyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one, two, or three oxo (when feasible), halogen, —OH, C1-C6 alkyl, or C1-C6 haloalkyl;
or Rc and Rd are taken together with the nitrogen atom to which they are attached to form a heterocycloalkyl optionally substituted with one, two, or three oxo, halogen, C1-C6 or C1-C6 haloalkyl; and wherein the cancer is selected from lung cancer, melanoma, breast cancer, ovarian cancer, colorectal cancer, gastric cancer, gallbladder cancer, prostate cancer, renal cancer, multiple myeloma, and lymphoma.
|