| CPC C07D 498/04 (2013.01) [C07D 401/06 (2013.01); C07D 401/14 (2013.01); C07D 405/14 (2013.01); C07D 409/14 (2013.01); C07D 413/06 (2013.01); C07D 413/14 (2013.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07D 487/04 (2013.01); C07D 491/048 (2013.01); C07D 491/107 (2013.01); C07D 495/04 (2013.01)] | 15 Claims |
|
1. A compound of formula (I):
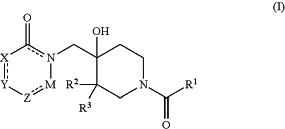 wherein
R1 is NRaRb or NRaCH2Rb, wherein Ra and Rb are independently selected from H, methyl, ethyl, propyl, CF3, optionally substituted cyclopropyl, optionally substituted cyclobutyl, optionally substituted cyclopentyl, optionally substituted cyclohexyl, optionally substituted phenyl, optionally substituted benzyl, optionally substituted pyridinyl, pyrazole, imidazole, furan, benzodioxol, optionally substituted oxadiazole, thiazole, and thiophene, wherein the optionally substituents are independently selected from halo, methyl, cyclopropyl and CN,
when R1 is NRaCH2Rb, the methylene group is substituted with CF3; or
when R1 is NRaRb, Ra and Rb together form an optionally substituted C3-C9 heterocycle together with the N to which they are attached;
R2 and R3 are independently selected from H, and C1-C6 alkyl, or together form an optionally substituted C3-C6 cycloalkyl or an optionally substituted C3-C6 heterocycloalkyl with the carbon to which they attached;
X is CR4a,
wherein R4a is selected from H, optionally substituted C1-C6 alkyl and halo;
Y is CR5,
wherein R5 is selected from H, halo, optionally substituted C1-C6 alkyl, optionally substituted C3-C6 cycloalkyl, optionally substituted C5-C8 aryl, optionally substituted C6-C9 arylalkyl, optionally substituted C3-C8 heteroaryl, CH2OH, NR′R′, NS(O)R′R″, SO2R′, C(O)R′, C(O)OR′, C(O)NR′R″, and OR′, wherein R′ and R″ are independently selected from H, C1-C6 alkyl, C5-C8 aryl, C6-C9 arylalkyl, and C3-C8 heteroaryl,
Z is N or CR7,
wherein R7 and is selected from H, halo, C1-C6 alkyl, C2-C6 alkene, C2-C6 alkyne, C3-C6 cycloalkyl, optionally substituted C3-C6 heterocycloalkyl, C5-C8 aryl, C6-C9 arylalkyl, C3-C8 heteroaryl, CN, C(O)ORc, CONRcRd, NRcRd, NS(O)RcRd, S(O)(Rc)NRd, SORc, SO2Rc, and SRc, wherein Rc and Rd are independently H, C1-C6 alkyl, C3-C6 cycloalkyl, C5-C6 aryl, C6-C9 arylalkyl, C3-C6 heteroaryl, CN, COOH, or COCH3 or Rc and Rd together form an optionally substituted C3-C7 heterocycle together with the heteroatom to which they are attached;
M is CH or C—CH3;
and
the ring including X, Y and Z is aliphatic or aromatic;
or a stereoisomer, tautomer, hydrate, N-oxide derivative or pharmaceutically acceptable salt thereof.
|