| CPC C07D 487/04 (2013.01) [C07D 207/16 (2013.01); C07D 211/60 (2013.01); C07D 401/06 (2013.01); C07D 401/10 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/12 (2013.01); C07D 409/04 (2013.01); C07D 409/14 (2013.01); C07D 413/12 (2013.01); C07D 413/14 (2013.01); C07D 417/12 (2013.01); C07D 471/04 (2013.01); C07D 495/04 (2013.01); C07F 9/6561 (2013.01); C07F 9/65583 (2013.01)] | 18 Claims |
|
1. A compound having the following Structure (I):
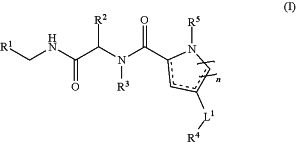 or a stereoisomer, tautomer, or pharmaceutically acceptable salt thereof, wherein:
R1 is a substituted aryl or a substituted or unsubstituted heteroaryl;
R2 is methyl;
R4 is a substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted cycloalkyl, or substituted or unsubstituted heterocyclyl;
R5 is hydrogen, alkyl, haloalkyl, cycloalkyl, phosphonalkyl, (CH2)mC(═O)OR6, C(═O)R6, C(═O)OR6, (CH2)mNR6S(O)2R7, or C(═O)NR6R7;
R6 and R7 are, at each occurrence, independently hydrogen, alkyl, haloalkyl, cycloalkyl, or arylalkyl;
L1 is a direct bond, —CR8aR8b—, —S(O)t—, NR8c, or —O—;
R8a and R8b are each independently hydrogen or alkyl, or R8a and R8b, together with the carbon to which they are attached form an optionally substituted 3-6 membered cycloalkyl;
R8c is hydrogen, alkyl, haloalkyl, (C═O)alkyl, (C═O)Oalkyl, (C═O)cycloalkyl, (C═O)Ocycloalkyl, (C═O)aryl, (C═O)Oaryl, (C═O)heteroaryl, (C═O)Oheteroaryl, (C═O)heterocyclyl, (C═O)O heterocyclyl, a substituted or unsubstituted aryl, a substituted or unsubstituted heteroaryl, a substituted or unsubstituted cycloalkyl, a substituted or unsubstituted heterocyclyl, a substituted or unsubstituted arylalkyl, a substituted or unsubstituted heteroarylalkyl, a substituted or unsubstituted cycloalkylalkyl, or a substituted or unsubstituted heterocyclylalkyl;
m is 1, 2, 3, 4, 5, or 6; and
t is 0, 1, or 2.
|