| CPC C07D 401/14 (2013.01) [C07B 2200/13 (2013.01)] | 20 Claims |
|
1. An isolated crystalline form of Compound 3:
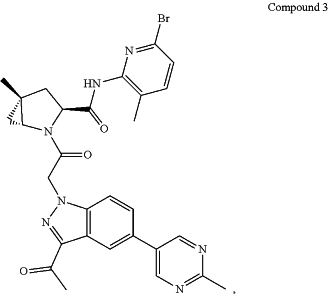 wherein the isolated crystalline form is:
Form B, characterized by a powder X-ray diffraction (PXRD) pattern comprising at least three 2theta values selected from 16.2±0.4°, 15.7±0.4°, 4.5±0.4°, 22.6±0.4°, 17.4±0.4°, 22.0±0.4°, 8.3±0.4°, 16.1±0.4°, 21.1±0.4°, 18.7±0.4°, 18.3±0.4°, 23.9±0.4°, and 27.5±0.4°; or
Form A, characterized by a powder X-ray diffraction (PXRD) pattern comprising at least one 2theta values selected from 2.6±0.4°, 3.6±0.4°, and 3.8±0.4°; or
Form M, characterized by a powder X-ray diffraction (PXRD) pattern comprising at least three 2theta values selected from 15.0±0.4°, 7.5±0.4°, 23.8±0.4°, 7.2±0.4°, 19.1±0.4°, 5.2±0.4°, 8.3±0.4°, 26.2±0.4°, 22.8±0.4°, 21.7±0.4°, and 24.9±0.4°.
|