| CPC C07D 401/14 (2013.01) [A61K 9/0019 (2013.01); A61K 9/0053 (2013.01); A61K 31/522 (2013.01); A61K 31/65 (2013.01); A61K 39/395 (2013.01); A61K 47/545 (2017.08); A61K 47/6809 (2017.08); A61P 25/28 (2018.01); A61P 43/00 (2018.01); C07D 217/26 (2013.01); C07D 401/12 (2013.01); C07D 495/04 (2013.01); C12N 15/115 (2013.01); C12Q 1/6816 (2013.01); G01N 33/5008 (2013.01)] | 16 Claims |
|
1. A compound of Formula I:
 or a pharmaceutically acceptable salt thereof; wherein:
Ligand is a small molecule RNA binder;
T1 is a bivalent tethering group selected from a C1-20 bivalent straight or branched hydrocarbon chain wherein 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 methylene units of the chain are independently and optionally replaced with a natural or non-natural amino acid, —O—, —C(O)—, —C(O)O—, —OC(O)—, —N(R)—, —C(O)N(R)—, —(R)NC(O)—, —OC(O)N(R)—, —(R)NC(O)O—, —N(R)C(O)N(R)—, —S—, —SO—, —SO2—, —SO2N(R)—, —(R)NSO2—, —C(S)—, —C(S)O—, —OC(S)—, —C(S)N(R)—, —(R)NC(S)—, —(R)NC(S)N(R)—, or -Cy-; and 1-20 of the methylene units of the chain are independently and optionally replaced with —OCH2CH2—;
wherein each -Cy- is independently a bivalent optionally substituted 3-8 membered saturated or partially unsaturated monocyclic carbocyclic ring, optionally substituted phenylene, an optionally substituted 4-8 membered saturated or partially unsaturated monocyclic heterocyclic ring having 1-3 heteroatoms independently selected from nitrogen, oxygen, or sulfur, an optionally substituted 5-6 membered monocyclic heteroaromatic ring having 1-4 heteroatoms independently selected from nitrogen, oxygen, or sulfur, an optionally substituted 8-10 membered bicyclic or bridged bicyclic saturated or partially unsaturated heterocyclic ring having 1-5 heteroatoms independently selected from nitrogen, oxygen, or sulfur, or an optionally substituted 8-10 membered bicyclic or bridged bicyclic heteroaromatic ring having 1-5 heteroatoms independently selected from nitrogen, oxygen, or sulfur;
each R is independently hydrogen or an optionally substituted group selected from C1-6 aliphatic, a 3-8 membered saturated or partially unsaturated monocyclic carbocyclic ring, phenyl, an 8-10 membered bicyclic aromatic carbocyclic ring, a 4-8 membered saturated or partially unsaturated monocyclic heterocyclic ring having 1-2 heteroatoms independently selected from nitrogen, oxygen, or sulfur, a 5-6 membered monocyclic heteroaromatic ring having 1-4 heteroatoms independently selected from nitrogen, oxygen, or sulfur, or an 8-10 membered bicyclic heteroaromatic ring having 1-5 heteroatoms independently selected from nitrogen, oxygen, or sulfur; and
Rmod is a photoactivatable group selected from
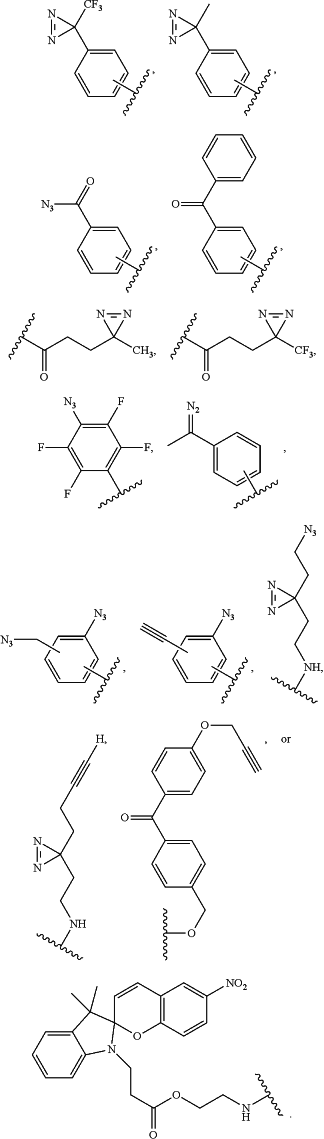 |