|
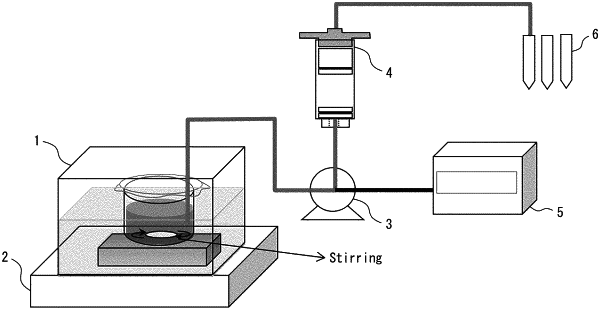
1. A blood purification device comprising a porous molded body comprising a porous molded body-forming polymer and an inorganic ion adsorbent, wherein the relationship B=−0.02 A+2.175±0.185 (74≤A≤94) is satisfied, where A is the water content and B is the bulk density of the porous molded body, while the number of microparticles with sizes of 10 μm or greater is no more than 25 and the number of microparticles with sizes of 25 μm or greater is no more than 3, in 1 mL of the physiological saline for injection at 3 months and 6 months after the physiological saline for injection has been encapsulated in the blood purification device.
|