| CPC A61K 31/4192 (2013.01) [A61K 31/19 (2013.01); A61K 31/194 (2013.01); A61K 31/351 (2013.01); A61K 31/381 (2013.01); A61K 31/382 (2013.01); A61K 31/415 (2013.01); A61K 31/4164 (2013.01); A61K 31/422 (2013.01); A61K 31/427 (2013.01); A61K 31/428 (2013.01); A61K 31/433 (2013.01); A61K 31/4439 (2013.01); A61K 31/454 (2013.01); A61K 31/4709 (2013.01); A61K 31/4725 (2013.01); A61K 31/497 (2013.01); A61K 31/501 (2013.01); A61K 31/506 (2013.01); A61K 31/5377 (2013.01); A61K 31/675 (2013.01); A61K 31/7048 (2013.01); A61K 38/51 (2013.01); A61K 45/06 (2013.01); C07D 231/14 (2013.01); C07D 233/90 (2013.01); C07D 249/06 (2013.01); C07D 401/04 (2013.01); C07D 401/10 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/04 (2013.01); C07D 403/10 (2013.01); C07D 403/14 (2013.01); C07D 405/04 (2013.01); C07D 405/10 (2013.01); C07D 405/14 (2013.01); C07D 413/10 (2013.01); C07D 417/04 (2013.01); C07D 417/10 (2013.01); C07F 9/65583 (2013.01); C12N 15/113 (2013.01); C12N 2310/14 (2013.01)] | 12 Claims |
|
1. A compound of formula III:
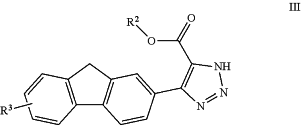 or a pharmaceutically acceptable salt, tautomer, stereoisomer, mixture of stereoisomers, or deuterated analog thereof, wherein:
R2 is hydrogen, —(CH2CH2O)1-9CH2CH2OCH3, C1-6 alkyl optionally substituted with one to three R4, cycloalkyl, or heteroaryl optionally substituted with one to three R5;
each R3 is independently aryl, heteroaryl, or heterocyclyl, wherein each R3 is optionally substituted with one to three R6;
each R4 is independently halo, hydroxy, —OC1-6alkyl, —NH2, —NHC1-6 alkyl, —N(C1-6alkyl)2, —OC(O)Ra, —OC(O)ORa, —OP(O)(ORb)2, or monocyclic heterocyclyl; wherein each is optionally substituted with one to three R5; provided only one R4 is heterocyclyl;
each R5 is independently cyano, halo, C1-4 alkyl, hydroxy, —OC1-4alkyl, C1-4 haloalkyl, or —OC1-4 haloalkyl;
each R6 is independently cyano, halo, —C(O)R7, —C(O)OR7, —C(O)NR7R8, —S(O)2NR7R8, —NR7C(O)R8, —OR7, C1-4 alkyl, —OC1-4alkyl, C1-4 haloalkyl, —OC1-4 haloalkyl, phenyl, heterocyclyl, or heteroaryl; wherein each is optionally substituted with one to three C1-4 alkyl, —C(O)OH or C1-4 haloalkyl;
R7 and R8 are each independently hydrogen, C1-4 alkyl or phenyl, pyridyl, or R7 and R8 together with the nitrogen atom to which they are attached form a heterocyclyl;
each Ra is independently C1-6 alkyl optionally substituted with —NH2, —NHC1-6 alkyl, —N(C1-6alkyl)2, or —OP(O)(ORb)2;
each Rb is independently hydrogen or C1-4 alkyl.
|