| CPC A61K 31/4439 (2013.01) [A61K 9/145 (2013.01); A61K 9/16 (2013.01); A61K 31/10 (2013.01); A61K 45/06 (2013.01); C07D 417/12 (2013.01); Y10T 428/2982 (2015.01)] | 32 Claims |
|
[ 23. Crystalline N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)-phenyl]acetamide mono methanesulfonic acid monohydrate of the following formula and having a purity of >99%:
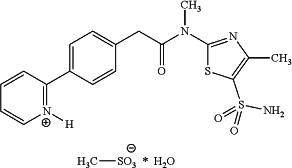 obtained by a process comprising the following steps
a) providing a mixture of an organic solvent and water containing N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)phenyl]-acetamide,
b) adding methanesulfonic acid at an elevated temperature to the mixture of step a to obtain a supersaturated solution of the mesylate of N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)phenyl]-acetamide,
c) optionally adding seed crystals of N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)phenyl]acetamide mono methane-sulfonic acid monohydrate at an elevated temperature between 30° C. and 90° C. to the supersaturated solution obtained in step b,
d) optionally stirring the supersaturated solution obtained in step b or step c,
e) cooling the supersaturated solution obtained in step b, step c or step d to room temperature,
f) filtering off the resulting crystals and washing the crystals with alcohol/water, and
g) optionally drying the crystals under vacuum at a temperature between 20° C. and 60° C. ]
|