| CPC H10K 85/342 (2023.02) [C07F 15/0033 (2013.01); C07F 15/0086 (2013.01); C09B 57/00 (2013.01); C09B 57/10 (2013.01); C09K 11/06 (2013.01); C09K 2211/1007 (2013.01); C09K 2211/1011 (2013.01); C09K 2211/1096 (2013.01); H10K 50/11 (2023.02); H10K 2101/10 (2023.02)] | 17 Claims |
|
1. A compound comprising a ligand L selected from the group A consisting of:
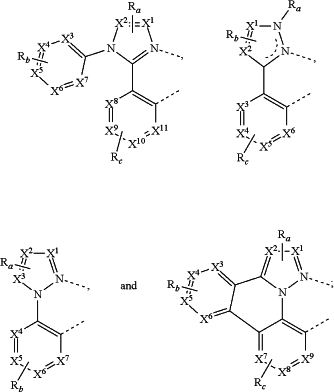 wherein each X1 to X11 are independently selected from the group consisting of carbon nitrogen;
wherein each Ra, Rb, and Rc may represent from mono substitution to the possible maximum number of substitution, or no substitution;
wherein each Ra, Rb, and Rc are independently selected from the group consisting of hydrogen, deuterium, halide, alkyl, cycloalkyl, heteroalkyl, arylalkyl, alkoxy, aryloxy, amino, silyl, alkenyl, cycloalkenyl, heteroalkenyl, alkynyl, aryl, heteroaryl, acyl, carboxylic acids, ester, nitrile, isonitrile, sulfanyl, sulfinyl, sulfonyl, phosphino, and combinations thereof; wherein when Ra, Rb, and Rc represent at least di substitution, each of the two adjacent Ra, two adjacent Rb, and two adjacent Rc are optionally fused or joined to form a ring;
wherein the ligand L is coordinated to a metal M;
wherein the ligand L is optionally linked with other ligands to form a tridentate, tetradentate, pentadentate or hexadentate ligand;
wherein the compound comprises at least one intramolecular hydrogen bonding interaction as shown in the scheme:
Z1—H—Z2, scheme 1;
wherein Z1 is a carbon of an alkyl group directly bonded to one of X1 to X11;
wherein Z2 is nitrogen and is one of X1 to X11; and
wherein in scheme 1, the proton NMR chemical shift of H is shifted downfield by at least 1.3 ppm compared to the compound when Z2 is carbon.
|