| CPC H01M 10/54 (2013.01) [B01J 6/001 (2013.01); B01J 6/002 (2013.01); B01J 8/008 (2013.01); B01J 19/06 (2013.01); B01J 19/2465 (2013.01); C22B 3/02 (2013.01); C22B 3/04 (2013.01); C22B 7/006 (2013.01); C22B 7/007 (2013.01); C22B 7/009 (2013.01); C22B 13/04 (2013.01); C22B 13/045 (2013.01); H01M 6/52 (2013.01); B01J 2208/00805 (2013.01); Y02P 10/20 (2015.11); Y02W 30/84 (2015.05)] | 20 Claims |
|
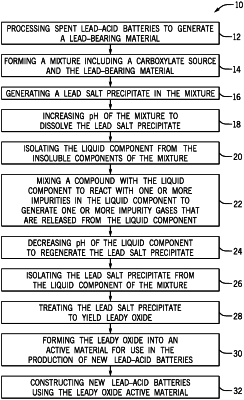
1. A method of forming a new lead-acid battery from recycling of a lead-acid battery comprising:
processing a spent lead-acid battery to generate a lead-bearing material;
forming a mixture comprising a carboxylate source and the lead-bearing material;
generating a lead salt precipitate in the mixture as the carboxylate source reacts with the lead-bearing material;
dissolving the lead salt precipitate;
isolating a liquid component of the mixture from one or more insoluble components of the mixture;
regenerating the lead salt precipitate;
isolating the regenerated lead salt precipitate from the liquid component of the mixture;
adding a hydride source to the liquid component;
treating the lead salt precipitate to yield a leady oxide;
forming a leady oxide active material with the leady oxide; and
forming a new lead-acid battery using the leady oxide active material.
|