| CPC G16H 10/40 (2018.01) [G16H 10/60 (2018.01); G16H 40/67 (2018.01); G16H 50/20 (2018.01)] | 20 Claims |
|
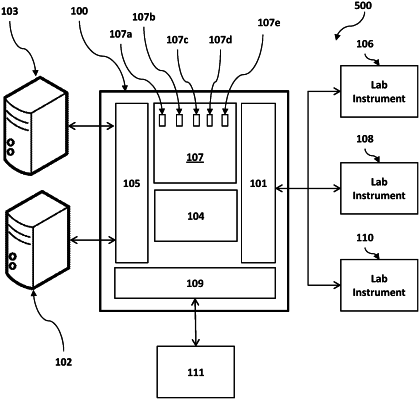
1. An information system for managing medical testing comprising:
a. a computing server on a communication path between at least one laboratory instrument and at least one external patient data information system, the at least one external patient data information system configured to receive patient data from one or more sources other than through the computing server;
b. an instrument communication interface communicatively coupled with the computing server and configured to provide a data communication between the computing server and the at least one external laboratory instrument;
c. an information system interface communicatively coupled with the computing server and configured to provide a further data communication between the computing server and the at least one external patient data information system, wherein the at least one external patient data information system is configured to maintain a patient data set comprising a set of test orders and a history data set;
wherein the computing server comprises:
i. a first receiving section configured to receive an instrument data set from the at least one external laboratory instrument via the instrument communication interface;
ii. a second receiving section configured to receive the history data set from the at least one external patient data information system via the information system interface;
iii. a test management section configured to build a test management data set based on the instrument data set received from the at least one external laboratory instrument and based on the history data set received from the at least one external patient data information system;
iv. a rules engine configured to apply a set of test management rules to the test management data set to generate a testing task; and
v. an updating section configured to update a set of test orders of a patient data set maintained by the at least one external patient data information system based upon the testing task;
wherein the instrument data set comprises test results associated with a patient; and
wherein the history data set comprises at least one of biographical and historical information associated with the patient; and
wherein the rules engine is configured to generate the testing task based upon a new piece of data in the test management data set, wherein the new piece of data in the test management data set is a result of a first test for the patient;
wherein the testing task is a test order cancellation of a second test for the patient;
wherein the rules engine is configured to determine whether to generate the test order cancellation based on whether the result of the first test for the patient renders the second test for the patient irrelevant; and
wherein the rules engine is configured to generate the test order cancellation of the second test for the patient based on determining that the result of the first test for the patient renders the result of the second test for the patient irrelevant.
|