| CPC C12Q 1/6886 (2013.01) [C12Q 2600/106 (2013.01); C12Q 2600/118 (2013.01); C12Q 2600/158 (2013.01); G01N 2800/52 (2013.01); G01N 2800/7028 (2013.01)] | 1 Claim |
|
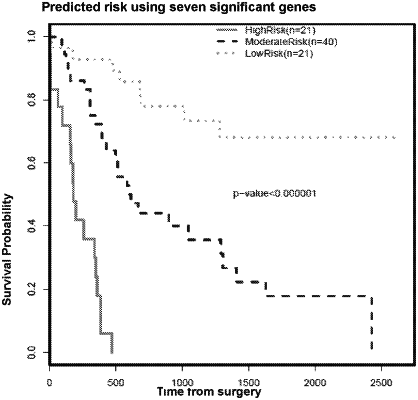
1. A method of treatment of gastroesophageal cancer in a human patient, said patient having had at least one perioperative treatment with one or more chemotherapeutic agents and having had surgical resection of a gastroesophageal tumour, the method comprising:
(a) measuring the gene expression of at least the genes CDH1, ELOVL5, EGFR, PIP5K1B, FGF1, CD44 and TBCEL in a sample obtained from the gastroesophageal tumour of the patient to obtain a sample gene expression profile of at least said genes CDH1, ELOVL5, EGFR, PIP5K1B, FGF1, CD44 and TBCEL; and
(b) deriving a risk score by weighting the measured, and optionally normalised, expression level of each gene and summing the weighted expression level of each of the genes, wherein the contribution to the total risk score made by CD44 and EGFR has the opposite sign to that of the contribution made by CDH1, ELOVL5, PIP5K1B, FGF1 and TBCEL;
(c) determining from said score that the patient is at high or moderate risk of poor prognosis; and
(d) following said determining administering therapy to the patient a therapeutically effective amount of at least one chemotherapeutic agent selected from the group consisting of epirubicin, cisplatin, 5-fluorouracil, capecitabine, oxaliplatin, and docetaxel.
|