| CPC C07D 471/04 (2013.01) [A61P 35/00 (2018.01); C07D 519/00 (2013.01)] | 20 Claims |
|
1. A compound of formula (I-h):
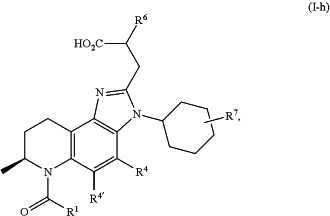 or a pharmaceutically acceptable salt thereof, wherein:
R1 is —C1-C6alkyl, —C2-C6alkenyl, —C2-C6alkynyl, —C3-C8cycloalkyl, —C4-C8cycloalkenyl, heterocyclyl, heteroaryl, aryl, —OR5, —N(R5)2, or —NHR5;
R4 and R4′ are each independently —H, halogen, —OH, —CN, or —NH2;
each R5 is independently —C1-C6alkyl, —C3-C8cycloalkyl, heterocyclyl, aryl, or heteroaryl;
R6 and R7 are each independently, at each occurrence, —C1-C6alkyl, —C3-C8cycloalkyl, —C4-C8cycloalkenyl, heterocyclyl, aryl, spirocycloalkyl, spiroheterocyclyl, heteroaryl, —OH, halogen, oxo, —CN, —SR8, —OR8, —(CH2)n—OR8, —NHR8, NR8R9, —S(O)2NR8R9, —S(O)2R8′, —C(O)R8′, —C(O)OR8, —C(O)NR8R9, —NR8C(O)R9′, —NR8S(O)2R9′, —S(O)R8′, —S(O)NR8R9, or —NR8S(O)R9′, wherein each alkyl, cycloalkyl, heterocyclyl, spirocycloalkyl, spiroheterocyclyl, heteroaryl, or aryl is optionally substituted with one or more R10;
R8 and R9 are each independently, at each occurrence, —H, —C1-C6alkyl, —C2-C6alkenyl, —C2-C6alkynyl, —C3-C8cycloalkyl, —C4-C8cycloalkenyl, heterocyclyl, aryl, heteroaryl, wherein each alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with one or more R10 or R11; or
R8 and R9 may combine with the atom to which they are both attached to form a —C3-C8cycloalkyl, —C4-C8cycloalkenyl, spirocycloalkyl, spiroheterocyclyl, heterocyclyl, heteroaryl, or aryl, wherein the formed —C3-C8cycloalkyl, —C4-C8cycloalkenyl, spirocycloalkyl, spiroheterocyclyl, heterocyclyl, heteroaryl, or aryl is optionally substituted with one or more R10 or R11;
R8′ and R9′ are each independently, at each occurrence, —C1-C6alkyl, —C2-C6alkenyl, —C2-C6alkynyl, —C3-C8cycloalkyl, —C4-C8cycloalkenyl, heterocyclyl, aryl, heteroaryl, wherein each alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with one or more R10 or R11; or
R8′ and R9′ may combine with the atom to which they are both attached to form a —C3-C8cycloalkyl, —C4-C8cycloalkenyl, spirocycloalkyl, spiroheterocyclyl, heterocyclyl, heteroaryl, or aryl, wherein the formed —C3-C8cycloalkyl, —C4-C8cycloalkenyl, spirocycloalkyl, spiroheterocyclyl, heterocyclyl, heteroaryl, or aryl is optionally substituted with one or more R10 or R11;
R10 and R11 are each independently, at each occurrence, —C1-C6alkyl, —C2-C6alkenyl, —C2-C6alkynyl, —C3-C8cycloalkyl, —C4-C8cycloalkenyl, heterocyclyl, heteroaryl, aryl, —OH, halogen, oxo, —NO2, —CN, —NH2, —OC1-C6alkyl, —OC3-C6cycloalkyl, —Oaryl, —Oheteroaryl, —NHC1-C6alkyl, —N(C1-C6alkyl)2, —S(O)2NH(C1-C6alkyl), —S(O)2N(C1-C6alkyl)2, —S(O)2C1-C6alkyl, —C(O)C1-C6alkyl, —C(O)NH2, —C(O)NH(C1-C6alkyl), —C(O)N(C1-C6alkyl)2, —C(O)OC1-C6alkyl, —N(C1-C6alkyl)SO2C1-C6alkyl, —S(O)(C1-C6alkyl), —S(O)N(C1-C6alkyl)2, or —N(C1-C6alkyl)S(O)(C1-C6alkyl), wherein each alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, heterocyclyl, heteroaryl, or aryl is optionally substituted with one or more —R12;
wherein any two R10 or any two R11, when on non-adjacent atoms, can combine to form a bridging cycloalkyl or heterocyclyl;
wherein any two R10 or any two R11, when on adjacent atoms, can combine to form a cycloalkyl, heterocyclyl, aryl or heteroaryl;
R12 is independently, at each occurrence, —C1-C6alkyl, —C2-C6alkenyl, —C2-C6alkynyl, —C3-C8cycloalkyl, —C4-C8cycloalkenyl, heterocyclyl, heteroaryl, aryl, —OH, halogen, oxo, —NO2, —CN, —NH2, —OC1-C6alkyl, —NHC1-C6alkyl, —N(C1-C6alkyl)2, —S(O)2NH(C1-C6alkyl), —S(O)2N(C1-C6alkyl)2, —S(O)2C1-C6alkyl, —C(O)C1-C6alkyl, —C(O)NH2, —C(O)NH(C1-C6alkyl), —C(O)N(C1-C6alkyl)2, —C(O)OC1-C6alkyl, —N(C1-C6alkyl)SO2C1-C6alkyl, —S(O)(C1-C6alkyl), —S(O)N(C1-C6alkyl)2, or —N(C1-C6alkyl)S(O)(C1-C6alkyl); and
n is an integer from 1 to 4.
|