| CPC C07D 471/04 (2013.01) [A61P 35/00 (2018.01); C07D 487/04 (2013.01); C07D 498/04 (2013.01); C07D 513/04 (2013.01); C07D 519/00 (2013.01); C12N 1/20 (2013.01); C12P 17/18 (2013.01); C12P 17/182 (2013.01); C12P 17/188 (2013.01)] | 2 Claims |
|
1. A pharmaceutical combination comprising:
one or more first active ingredients of general formula (I)
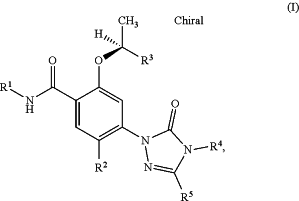 in which
R1 represents a group selected from
a C1-C8-alkyl group, which is optionally substituted with a group selected from
C3-C8-cycloalkyl, phenyl and monocyclic or bicyclic heteroaryl,
wherein said phenyl substituent is optionally substituted, one, two or three times with one or more substituents independently selected from a halogen atom, a C1-C3-alkyl group, a C1-C4-haloalkyl group, a C1-C3-alkoxy group and a hydroxy group,
a C2-C8-haloalkyl group,
a C3-C8-cycloalkyl group, which is optionally substituted, one or two times, each substituent independently selected from a halogen atom, a hydroxy group, a phenyl group and a —N(R7)(R8) group,
wherein said phenyl substituent is optionally substituted, one, two or three times, each substituent independently selected from a halogen atom, a C1-C3-alkyl group, a C1-C4-haloalkyl group, a C1-C3-alkoxy group and a hydroxy group,
a C2-C6-cyanoalkyl group,
a C2-C6-hydroxyalkyl group,
a (C2-C6-hydroxyalkyl)-O—(C2-C6-alky)- group,
a —(C2-C6-alkyl)-N(R7)(R8) group,
a —(C1-C6-alkyl)-C(═O)N(R7)(R8) group,
a 4- to 7-membered, optionally unsaturated, heterocyclic group, which is connected to the rest of the molecule via a carbon atom, and which is optionally substituted one or two times, each substituent independently selected from a C1-C3-alkyl group, a 5- to 6-membered heteroaryl group, a-C(═O)O(C1-C4-alkyl) group, a —C(═O)(C1-C6-alkyl) group, a —C(═O)(C3-C6-cycloalkyl) group, a —S(═O)2(C1-C6-alkyl) group and a oxo (═O) group,
wherein said 5- to 6-membered heteroaryl substituent is optionally substituted, one, two or three times, each substituent independently selected from a halogen atom or a group selected from C1-C3-alkyl, C1-C4-haloalkyl, C1-C3-alkoxy and hydroxy,
a phenyl group, which is optionally substituted, one, two, three, four or five times, each substituent independently selected from a halogen atom or a group selected from C1-C6-alkyl, C3-C8-cycloalkyl, C1-C6-haloalkyl, C1-C6-hydroxyalkyl, C2-C6-alkenyl, C2-C6-alkynyl, aryl, —(C1-C6-alkyl)-aryl, -aryl-(C1-C6-alkyl), C1-C6-alkoxy, —O(C2-C6-alkenyl), C1-C6-haloalkoxy, C3-C8-cycloalkoxy, hydroxy, aryl, —O-aryl, cyano, —C(═O)OR6, —C(═O)N(R7)(R8), —N(R7)(R8), —(C1-C6-alkyl)-N(R7)(R8), —(C1-C6-alkyl)-C(═O)OR6, —(C1-C6-alkyl)-C(═O)N(R7)(R8),), —SH, —S—(C1-C6-alkyl), —S—(C2-C6-alkenyl), —S(═O)2N(R7)(R8), —S(═O)2(C1-C6-alkyl), —S(═O)2—O—(C2-C6-alkenyl), —S(═O)(═NR14)(C1-C3-alkyl), —N(O)2, —P(═O)(C1-C3-alkyl)2 and SF5,
or wherein two vicinal substituents of said phenyl groups may form together a 5- or 6-membered, optionally heterocyclic, aromatic or non-aromatic ring, having optionally 1-3 heteroatoms independently selected from —N═, —NH—, —N(R7)—, —O—, —S—, and optionally containing a C(═O) group, and wherein the so formed ring is optionally substituted one or two times, each substituent independently selected from a halogen atom or a group selected from
C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-alkynyl, aryl, —(C1-C6-alkyl)-aryl, -aryl-(C1-C6-alkyl), C3-C8-cycloalkyl, C1-C6-alkoxy, —O(C2-C6-alkenyl), C1-C6-haloalkoxy, C3-C8-cycloalkoxy, aryl, —O-aryl, cyano, —C(O)OH, hydroxy, —SH, —S—(C1-C6-alkyl), —S—(C2-C6-alkenyl), —S(═O)2(C1-C6-alkyl), —N(O)2, and —N(R7)(R8) and
a monocyclic or bicyclic heteroaryl group which is optionally substituted one, two or three times, each substituent independently selected from a halogen atom or a group selected from
C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-alkynyl, —(C1-C6-alkyl)-aryl, -aryl-(C1-C6-alkyl), C3-C8-cycloalkyl, C1-C6-alkoxy, —O(C2-C6-alkenyl), C1-C6-haloalkoxy, C3-C8-cycloalkoxy, cyano, —C(═O)OR6, hydroxy, —SH, —S—(C1-C6-alkyl), —S—(C2-C6-alkenyl), —S(═O)2(C1-C6-alkyl), —N(O)2, and —N(R7)(R8),
R2 represents a hydrogen atom or a halogen atom,
R3 represents a group selected from
a C1-C6-alkyl group,
a C3-C8-cycloalkyl group,
a C1-C6-haloalkyl group,
a C1-C6-hydroxyalkyl group,
a C2-C6-alkenyl group,
a C2-C6-alkinyl group,
a C4-C8-cycloalkenyl group,
a (C1-C6-alkyl)-N(R7)R8 group,
a —(C1-C6-alkyl)-N(H)C(═O)R6 group,
a —(C1-C6-alkyl)-N(H)C(═O)OR15 group,
a —(C1-C6-alkyl)-(4- to 7-membered nitrogen containing heterocycloalkyl) group, wherein said 4- to 7-membered nitrogen containing heterocycloalkyl group is connected to the alkyl group via a carbon atom of the heterocycloalkyl group and wherein said 4- to 7-membered nitrogen containing heterocycloalkyl group is optionally substituted with a C1-C3-alkyl group,
and
a phenyl group,
which is optionally substituted, one, two or three times, each substituent independently selected from a halogen atom or a group selected from C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-alkynyl, aryl, —(C1-C6-alkyl)-aryl, -aryl-(C1-C6-alkyl), C3-C8-cycloalkyl, C1-C6-alkoxy, —O(C2-C6-alkenyl), C1-C6-haloalkoxy, C3-C8-cycloalkoxy, aryl, —O-aryl, cyano, —C(═O)OR6, hydroxy, —SH, —S—(C1-C6-alkyl), —S—(C2-C6-alkenyl), —S(═O)2(C1-C6-alkyl), —N(O)2, and —N(R7)(R8),
R4 and R5 jointly form an optionally unsaturated, heterocyclic ring A of partial formula (i)
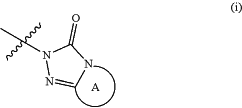 whereby ring A in addition to the two mandatory atoms, the nitrogen atom and the carbon atom bridging the two rings, bears additional 3 to 6 members selected from —O—, —S—, —S(═O)—, —S(═O)2—, —S(═O)(═NR14)—, —N═, —N(R7)—, —C(═O)—, —C(═O)—, —CH═, —CR11═, —C(R12)2—, —C(H)(R13)—,
R6 represents a hydrogen atom or a group selected from
a C1-C6-alkyl group and a benzyl group,
R7 and R8 represent, independently from each occurrence, a hydrogen atom or a group selected from
a C1-C6-alkyl group, a C2-C6-alkenyl group, a C2-C6-hydroxyalkyl group, a haloalkyl group, a aryl group, a (C1-C6-alkyl)-aryl group, and a —(C2-C6-alkyl)-N(R9)(R10) group, or
R7 and R8 together with the nitrogen to which they are attached represent a
nitrogen containing 4- to 7-membered heterocycloalkyl group,
wherein said 4- to 7-membered nitrogen containing heterocycloalkyl group is optionally substituted with a group selected from
C1-C6-alkyl, —S—(C1-C6-alkyl), —S—(C2-C6-alkenyl), —S(═O)2(C1-C3-alkyl), —S(═O)2—(C2-C6-alkenyl), and —C(═O)OR6 —,
R9 and R10 represent, independently from each occurrence, a hydrogen atom or a C1-C3-alkyl group,
or
R9 and R10 together with the nitrogen to which they are attached represent a
nitrogen containing 4- to 7-membered heterocycloalkyl group,
R11 represents, independently from each occurrence a hydrogen atom, a halogen atom or a group selected from
C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-alkynyl, aryl, —(C1-C6-alkyl)-aryl, -aryl-(C1-C6-alkyl), C3-C8-cycloalkyl, C1-C6-alkoxy, —O(C2-C6-alkenyl), C1-C6-haloalkoxy, C3-C8-cycloalkoxy, aryl, —O-aryl, cyano, —C(═O)OR6 —, hydroxy, —SH, —S—(C1-C6-alkyl), —S—(C2-C6-alkenyl), S(═O)2(C1-C6-alkyl), —S(═O)2—(C2-C6-alkenyl), —N(O)2, and —N(R7)(R8)
R12 represents, independently from each occurrence, a hydrogen atom, a halogen atom or a C1-C3-alkyl group,
R13 represents a group selected from
C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-alkynyl, aryl, —(C1-C6-alkyl)-aryl, -aryl-(C1-C6-alkyl), C3-C8-cycloalkyl, C1-C6-alkoxy, —O(C2-C6-alkenyl), C1-C6-haloalkoxy, C3-C8-cycloalkoxy, aryl, —O-aryl, cyano, —C(═O)OR6 —, hydroxy, —SH, —S—(C1-C6-alkyl), —S—(C2-C6-alkenyl), S(═O)2—(C1-C6-alkyl), —S(═O)2—(C2-C6-alkenyl), —N(O)2, and —N(R7)(R8),
R14 represents a hydrogen atom or a group selected from
a cyano group and a —C(═O)(C1-C3-haloalkyl) group,
R15 represents a group selected from
a C1-C6-alkyl group and a benzyl group,
or a tautomer, an N-oxide, or a salt thereof, or a salt of a tautomer or an N-oxide of said compound, and
one or more further active ingredients.
|