| CPC C07D 471/04 (2013.01) [C07C 291/00 (2013.01); C07D 471/20 (2013.01); C07D 487/10 (2013.01); C07D 493/04 (2013.01); C12N 9/22 (2013.01); C12Q 1/6837 (2013.01); G01N 33/582 (2013.01)] | 3 Claims |
|
1. A method of inhibiting the activity of an RNA guided endonuclease-guide RNA complex, the method comprising contacting the RNA guided endonuclease-guide RNA complex with a small molecule;
wherein the small molecule is a compound having the formula of Formula I:
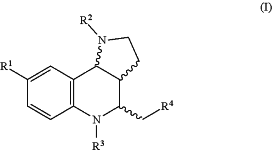 wherein R1 is independently selected at each occurrence from hydrogen, —X, —R, -L1-X, or -L1-R; and
R2-R4 are independently selected from hydrogen, —X, —R, -L1-X, or -L1-R; where
X is independently selected at each occurrence from CN, OH, CF3, COOH, OR, NR2, or halogen;
L1 is selected from —(CH2)n—, —(CH2)n—C(O)O—, —(CH2)n—C(O)—NH—, —C(O)—NH—(CH2)n—, —(CH2)n—NH—C(O)—, —(CH2)n—NH—SO2—, —NH—SO2—(CH2)n—, —(CH2)n—SO2—NH—, —(CH2)n—SO2—, —(CH2)n—SO2—NH—C(O)—, —(CH2)n—RL—, —RL—C(O)—O—, —RL—NH—C(O)—(CH2)n—, —RL—NH—S(O)2—(CH2)n—, —S—, —S(O)—, —S(O)2—; wherein n is independently at each occurrence 0, 1, 2, 3, 4, 5, or 6;
RL is independently selected at each occurrence from C1-C12 linear and/or branched and/or cyclic and/or aromatic bivalent radicals; optionally substituted with one or more groups X and/or with 1-6 heteroatoms selected from O, S, N, P, F, Cl, Br, I; and
R is independently selected at each occurrence from C1-12 hydrocarbons, optionally substituted with one or more groups selected from CN, OH, CF, COOH, and NH2, and/or with 1-10 heteroatoms selected from O, S, N, P, F, Cl, Br, I, and combinations thereof.
|