| CPC C07D 403/14 (2013.01) [A61P 3/10 (2018.01); C07D 239/95 (2013.01); C07D 403/06 (2013.01); C07D 403/12 (2013.01); C07D 413/12 (2013.01); C07D 413/14 (2013.01)] | 22 Claims |
|
1. A compound of formula
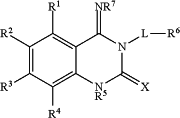 or a pharmaceutically acceptable salt, tautomer or stereoisomer thereof,
wherein:
X is S or O;
R1 is selected from H, hydroxyl, halo, C1-6alkyl, C1-4alkoxy, O-CH2phenyl, O-phenyl;
R2 is selected from H, hydroxyl, halo, C1-6alkyl, C1-4alkoxy, O-CH2phenyl, O-phenyl;
R3 is selected from H, hydroxyl, halo, C1-6alkyl, C1-4alkoxy, O-CH2phenyl, O-phenyl;
R4 is selected from H, hydroxyl, halo, C1-6alkyl, C1-4alkoxy, O-CH2phenyl, O-phenyl;
or R1 and R2, or R2 and R3, or R3 and R4 together form C1-3alkylenedioxy;
R5 is selected from H and C1-6 alkyl;
L is selected from C1-6 alkylene-indolyl, C1-6alkylene-NHC(O)—, azetidinyl-C(O)—, C1-6alkylene-NHC(O)-indolyl, and C1-6alkylene-NHSO2—;
R6 is selected from H, halo, hydroxyl, C1-6alkyl, C2-6alkenyl, C2-6alkynyl, C6-10aryl optionally substituted with 1 or 2 RX groups, C1-9heteroaryl optionally substituted with 1 or 2 RX groups, C2-5heterocycloalkyl optionally substituted with 1 or 2 RX groups, or is absent;
each RX is independently selected from hydroxyl, halo, nitro, NR′R″; C1-4alkyl, C3-6cycloalkyl, haloC1-4alkyl, C1-4alkoxy, C6-10aryl optionally substituted with 1 or 2 RY groups, C1-9heteroaryl, C1-4alkyl-(C1-9heteroaryl), C(O)OC1-4alkyl, C(O)NHRY, C2-5heterocycloalkyl optionally substituted with 1 or 2 C1-4alkyl groups, C(O)—(C2-5heterocycloalkyl) optionally substituted with 1 or 2 C1-4alkyl groups, or two adjacent RX groups together form C1-3alkylenedioxy;
RY is selected from H, hydroxyl, halo, C1-4 alkyl, haloC1-4 alkyl, C1-4 alkoxy, C1-4 alkylheterocycloalkyl, C(O)—(C1-4 alkylC2-5heterocycloalkyl), C1-4alkylNR′R″;
R′ and R″ are independently selected from H, C1-4alkyl, C1-4 alkylC2-5heterocycloalkyl;
R7 is selected from H, C1-4alkyl, C1-6alkylC1-9heteroaryl,
wherein the term heterocycloalkyl refers to a cycloalkyl ring in which one or more carbon atoms has been replaced by one or more heteroatoms.
|