| CPC C07D 215/42 (2013.01) [C07D 215/44 (2013.01); C07D 215/46 (2013.01); C07D 401/10 (2013.01); C07D 401/12 (2013.01); C07D 405/10 (2013.01); C07D 405/12 (2013.01); C07D 409/10 (2013.01); C07D 413/12 (2013.01)] | 20 Claims |
|
1. A compound having a structure represented by a formula:
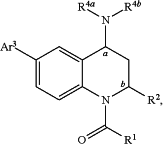 wherein R1 is selected from C1-C4 alkyl, C1-C4 haloalkyl, and C1-C4 deuterated alkyl;
wherein R2 is C1-C4 alkyl;
wherein R4a is selected from hydrogen, C1-C4 alkyl, and amine protecting group;
wherein R4b is selected from —(CH2)nCy1, —(CH2)oAr2, and amine protecting group;
wherein each of n and o, when present, is selected from 0, 1, 2, and 3;
wherein Cy1, when present, is selected from cycloalkyl, five-membered heterocycle, and six-membered heterocycle and is substituted with 0-4 non-hydrogen groups independently selected from halogen, —OH, —CN, C1-C4 alkyl, C1-C4 alkoxy, and C1-C4 haloalkyl;
wherein Ar2, when present, is selected from aryl and 5- to 12-membered heteroaryl and is substituted with 0-4 R5 groups independently selected from halogen, —OH, —CN, —NO2, C1-C4 alkyl, C1-C4 alkoxy, C1-C4 haloalkyl, C1-C4 haloalkoxy, C1-C4 aminoalkyl, C3-C6 cycloalkyl, —COR21, —CO2R21, —CONR22aR22b, (CH2)qNR22aR22b, —SO2NR22aR22b, —NR23C(O)R24, —NR23(CH2)q(C3-C6 cycloalkyl), —NR23(CH2)q(heterocycloalkyl), and 3- to 5-membered heterocycloalkyl;
wherein q, when present, is selected from 0, 1, 2, 3, and 4;
wherein each occurrence of R21, when present, is independently selected from hydrogen, C1-C6 alkyl, C3-C6 cycloalkyl, C3-C6 heterocycloalkyl, —COR30, —(C1-C4 alkyl)OC(O)(C1-C4 alkyl), and —(C1-C6 alkyl)NHC(O)A;
wherein A has a structure:
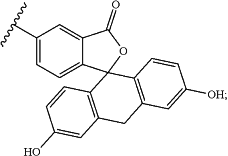 wherein each occurrence of R30, when present, is independently selected from hydrogen, C1-C6 alkyl, C1-C6 alkoxy, C1-C6 aminoalkyl, C3-C6 cycloalkyl, and C3-C6 heterocycloalkyl;
wherein each occurrence of each of R22a and R22b, when present, is independently selected from hydrogen, C1-C6 alkyl, C3-C6 cycloalkyl, C3-C6 heterocycloalkyl, and —COR30;
wherein R23, when present, is selected from hydrogen and C1-C4 alkyl;
wherein R24, when present, is selected from C1-C4 alkyl, C1-C4 hydroxyalkyl, C1-C4 alkyl(C1-C4 alkoxy), C1-C4 alkylamino, (C1-C4)(C1-C4) dialkylamino, C1-4 alkylamino(C1-C4 alkyl), (C1-C4)(C1-C4) dialkylamino(C1-C4 alkyl), —(CH2)r(C3-C6 cycloalkyl), and —(CH2)r(C3-C6 heterocycloalkyl);
wherein r, when present, is selected from 0, 1, 2, and 3;
wherein Ar3 is:
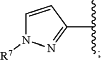 wherein R7, when present, is selected from hydrogen, C1-C4 alkyl, and aryl substituted with 0-4 non-hydrogen groups independently selected from halogen, —OH, —CN, C1-C4 alkyl, C1-C4 alkoxy, and C1-C4 haloalkyl;
provided that when R7 is hydrogen or methyl, then R4b is not amine protecting group, and
provided that when o is 0, then Ar2 is substituted with at least one non-hydrogen group,
or a pharmaceutically acceptable salt thereof.
|