| CPC C07D 209/10 (2013.01) [A61K 49/006 (2013.01); A61K 49/0032 (2013.01); A61K 49/0058 (2013.01); C07D 403/12 (2013.01); C09K 11/06 (2013.01); G01N 21/6428 (2013.01); G01N 33/533 (2013.01); G01N 33/582 (2013.01); G01N 2021/6439 (2013.01)] | 14 Claims |
|
1. A method for in vivo visualization of a renal system, comprising:
(i) administering to the subject a pharmaceutical composition comprising a fluorescence agent comprising a compound of Formula IA in an amount sufficient to effect fluorescence detection of the portion of the renal system upon irradiation with light having a wavelength in the range of 650 nm to 900 nm:
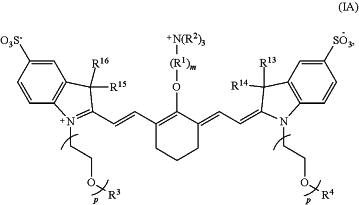 wherein
R1 is —CH2—,
each R2 independently is methyl, ethyl, n-propyl, or isopropyl,
R3 and R4 independently are C1-C10 alkyl,
R13 R16 independently are C1-C10 alkyl,
m is 3, and
p is 2, 3, or 4;
(ii) allowing time following the administration for accumulation of the compound of Formula IA in the renal system;
(iii) irradiating the renal system with light having a wavelength in the range of 650 nm to 900 nm (NIR) to produce a fluorescence signal of the compound of Formula IA in the renal system;
iv) visualizing at least one portion of the renal system by detection of the fluorescence signal produced upon the irradiation (iii);
wherein
a contrast to background ratio (CBR) of the fluorescence signal to a background signal in a region of the renal system being visualized is 1.5 or more within 20 minutes of the administration.
|